Figure 4. Phosphorylation of Bim on Ser65 and Thr112 in vivo.
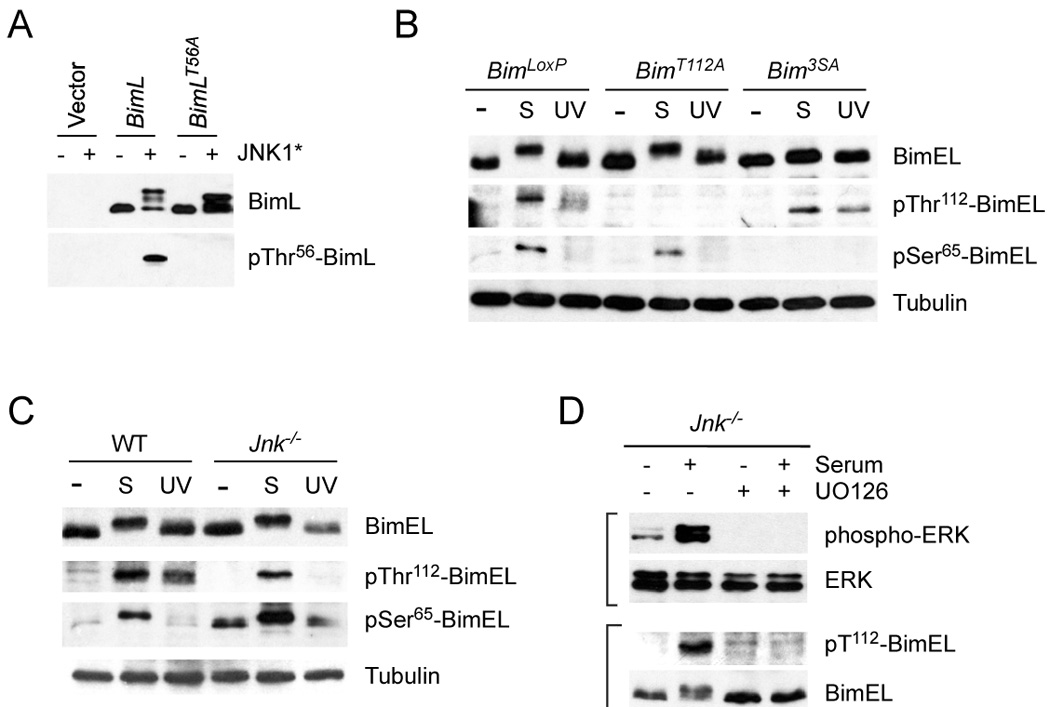
A) Characterization of an antibody to the Bim phosphorylation site Thr112 on BimEL (Thr56 on BimL). 293T cells were transfected with an empty expression vector or a vector that expresses BimL. The expression of BimL and phosphoThr56-BimL was detected by immunoblot analysis of cells extracts. The effect of co-expression of constitutively activated JNK1 (JNK1*) and the replacement of Thr56 with an Ala residue is shown.
B) Homozygous BimLoxP, BimT112A, and Bim3SA primary MEF were serum-starved or treated with serum (S) (10% fetal bovine serum; 30 mins) or UV light (60 J/m2; 60 mins). Cell extracts were examined using antibodies to Bim, phospho-Thr112-Bim, phosphoSer65-Bim, and Tubulin.
C) Wild-type and Jnk1−/− Jnk2−/− (Jnk−/−) fibroblasts were serum-starved or treated with serum (S) or UV light. Cell extracts were examined using antibodies to Bim, phospho- Thr112-Bim, phosphoSer65-Bim, and Tubulin. The basal phosphorylation of BimEL on Ser65 was slightly increased in JNK-deficient fibroblasts compared with wild-type fibroblasts.
D) Jnk1−/− Jnk2−/− (Jnk−/−) fibroblasts were serum-starved or treated with serum and/or the MEK inhibitor U0126 (30 mins). Cell extracts were examined using antibodies to phospho-ERK, ERK, phospho-Thr112-Bim, and Bim.
