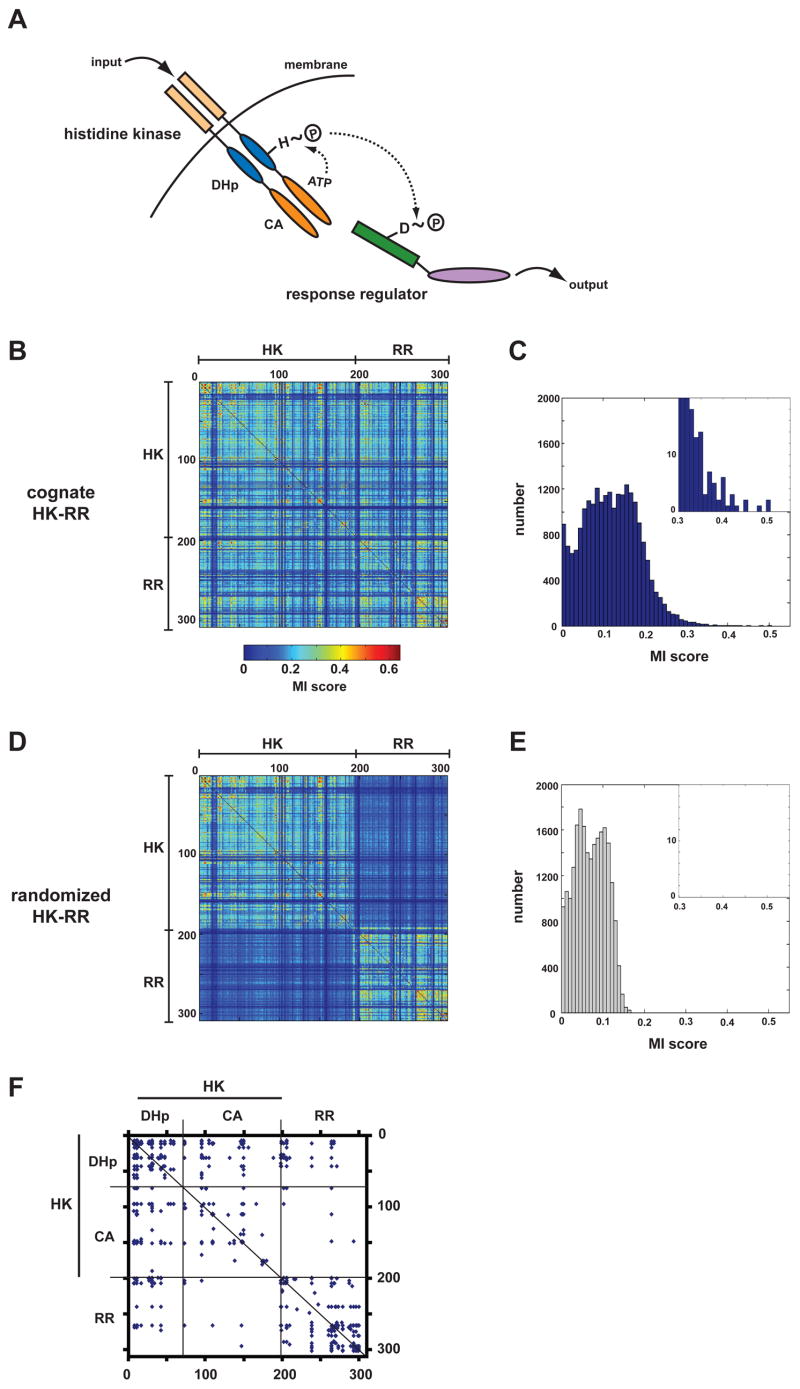
Figure 1. Mutual information analysis of two-component signal transduction proteins.
(A) Schematic of the two-component signaling paradigm. Receipt of a signal stimulates autophosphorylation of a histidine kinase (HK). The phosphoryl group is subsequently passed to a cognate response regulator (RR), which can trigger changes in gene expression or other physiological processes. (B) Identification of covarying residues in histidine kinases and response regulators. Cognate, operon pairs of histidine kinases and response regulators were concatenated and aligned. Using this multiple sequence alignment, every pair of positions was examined for covariation using an analysis of mutual information. The matrix of scores for each pair of positions in the alignment is plotted as a heatmap, according to the color legend shown. Positions within the alignment corresponding to the histidine kinase and the response regulator are indicated. (C) Histogram of interprotein (HK-RR) mutual information scores from panel B. (D) The pairings of histidine kinases and response regulators from panel B were randomized and the mutual information analysis repeated. The heatmap of scores shows that the randomization process reduces interprotein, but not intraprotein (HK-HK, RR-RR), covariation. (E) Histogram of interprotein mutual information scores for the alignment in which HK-RR pairings were scrambled. (F) For the multiple sequence alignment containing cognate HK-RR pairs, the graph indicates the pairs of amino acids with scores >0.35. Regions of the alignment corresponding to the HK and RR are indicated as well as the two HK domains, the dimerization and histidine phosphotransfer (DHp) domain and the catalytic and ATP-binding (CA) domain (see panel A).