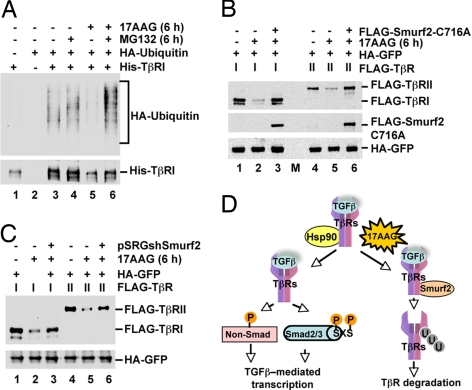
Fig. 6.
Smurf2 is essential for 17AAG-induced TβR degradation. (A) 17AAG increases TβRI ubiquitination. HEK293T cells were cotransfected with His-TβRI and HA-ubiquitin and treated for 6 h with 17AAG and/or MG132 as indicated. His-TβRI was precipitated with Ni-NTA agarose, and TβRI ubiquitination was determined by anti-His and anti-ubiquitin immunoblotting. (B) Dominant-negative Smurf2(C716A) prevents 17AAG-induced TβR degradation. HEK293T cells were cotransfected with FLAG-TβR, HA-GFP, and FLAG-Smurf2(C716A) as indicated. Cells were treated with 2 μM 17AAG for 6 h as specified and harvested for Western blotting with anti-FLAG and anti-HA antibodies. M, molecular weight marker lane. (C) Smurf2 depletion reduces 17AAG-induced TβR degradation. HEK293T cells were cotransfected with FLAG-TβR, HA-GFP, and shSmurf2 as indicated. Cells were treated and Western blotting was performed as described in B. (D) Working model for the regulation of TGFβ signaling by Hsp90. Hsp90 protects TβRs from degradation, ensuring that activated receptors promote TGFβ-mediated transcription via Smad and non-Smad targets. Inhibition of Hsp90 by 17AAG promotes Smurf2-mediated TβR-ubiquitination and degradation, abolishing TGFβ-mediated transcription.