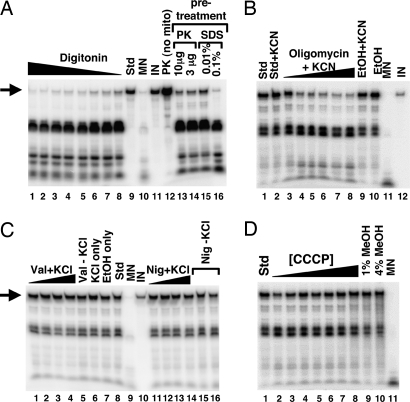
Fig. 6.
tRNA import occurs by a mechanism distinct from protein import. (A) The import of tRNACUGGln was examined in purified mitochondria pretreated with digitonin (at 0.01, 0.1, 0.5, 1, and 5% final concentration; lanes 1–8, respectively) and compared with a standard import reaction (Std, lane 9) without pretreatment. In addition, mitochondria were pretreated with proteinase K (PK, lanes 13 and 14) or mildly treated with SDS (lanes 15 and 16). PK (no mito), incubation of the labeled transcript in the presence of an amount of proteinase K equivalent to that used in the assay in lane 13 but in the absence of mitochondria, demonstrating that the lack of protection in lanes 13 and 14 cannot be ascribed to degradation of the tRNA by proteinase K. (B) Mitochondria were also treated with increasing concentrations of oligomycin (1.3, 3.2, 31.7, 63.3, 95, and 126 nM; lanes 3–8, respectively) to inhibit ATPase activity in the presence of KCN. Import of tRNA under these conditions was compared with a standard import reaction (Std, lane 1) and a standard reaction with KCN alone (lane 2). In addition, tRNAs were also imported into mitochondria treated with ethanol (EtOH) (lane 10) or KCN + ethanol (EtOH+KCN, lane 9) alone serving as a control for the various cofactors and solvents. (C) Mitochondria were also treated with increasing concentrations of the potassium ionophore valinomycin (1, 5, 10, and 20 μM; lanes 1–4, respectively) in the presence of KCl (70 mM final concentration). The highest level of valinomycin used represents 100× the concentration required to collapse the membrane potential (data not shown). Val+KCl (lane 5), KCl only (lane 6), and EtOH only (lane 7) refer to import reactions in the presence of these various components used as cofactors or solvents in the valinomycin experiment. Incubation of tRNA alone with any of these components does not inhibit MN cleavage (data not shown). Similar reactions were performed in reactions where valinomycin was replaced by nigericin (Nig) (1, 5, 10, and 20 μM; lanes 11–14), a potent inhibitor of the pH gradient. nig (−) KCL (lanes 15 and 16) refers to an import reaction in the presence of nigericin but in the absence of KCl. Control reactions without mitochondria and in the presence of nigericin showed that nigericin does not inhibit MN cleavage (data not shown). (D) tRNA import assays with mitochondria treated with the uncoupler CCCP at concentrations well above what is required to destroy the electrochemical gradient (0.1, 0.25, 0.5, 0.75, 1.0, 1.5, and 2 mM; lanes 2–8, respectively). Because CCCP is dissolved in methanol, control import reactions in the presence of methanol are also shown (lanes 9 and 10). Throughout the figure, arrows denote the full-length tRNA substrate, and the black triangle represents increasing or decreasing concentrations of a given chemical. MN and IN, control reactions for nuclease cleavage and as a quantitation standard, respectively.