Abstract
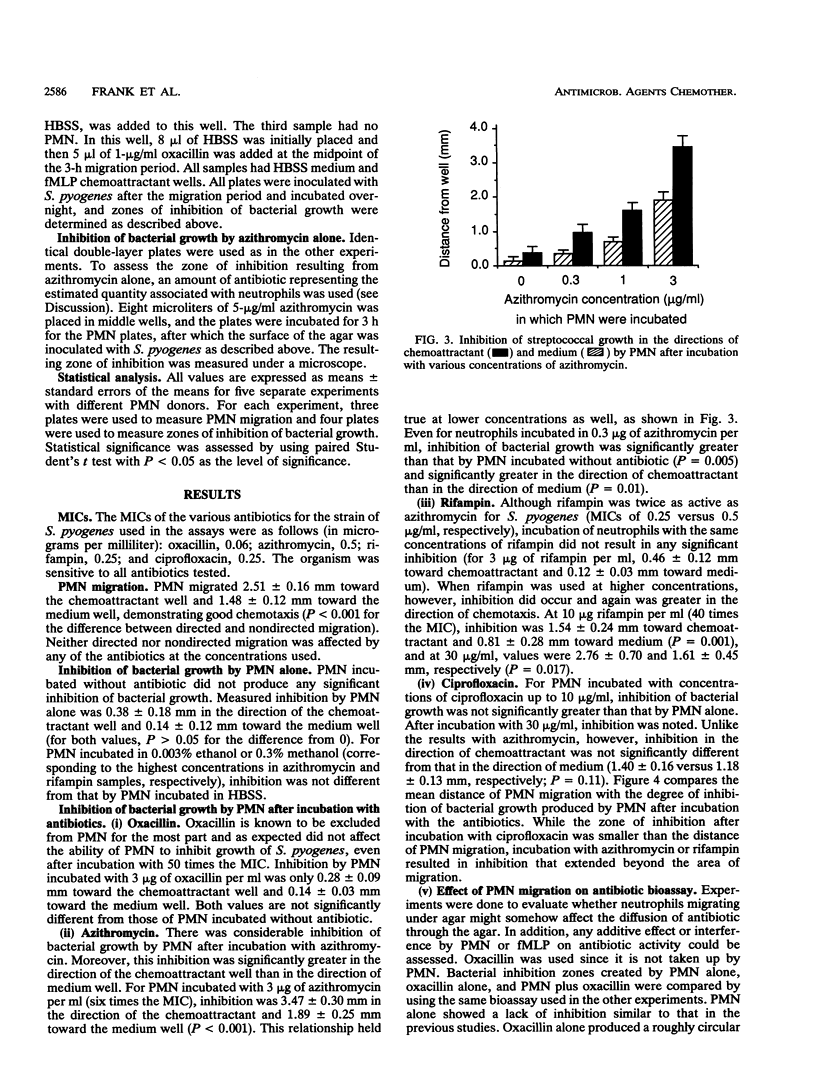
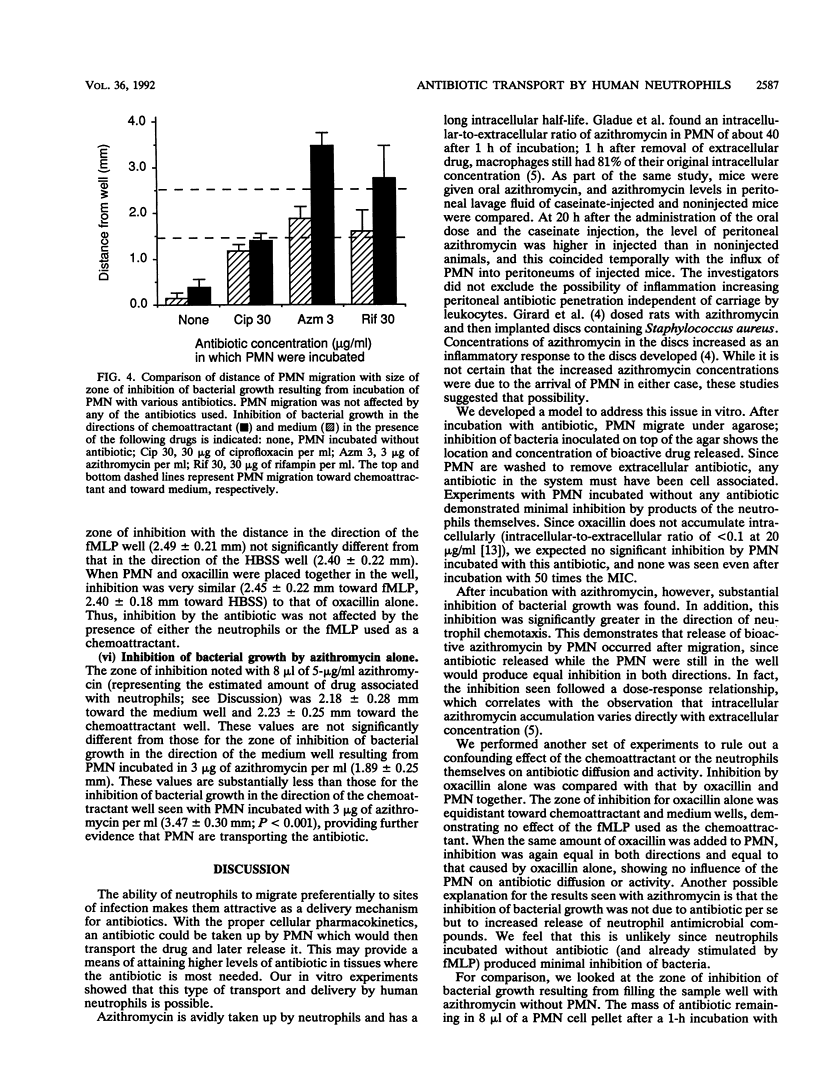
Several antibiotics are concentrated inside polymorphonuclear leukocytes (PMN). To investigate whether PMN could act as vehicles for delivery of antibiotics, we combined an assay measuring PMN chemotaxis under agarose with a bioassay measuring levels of antibiotic in agar. Double-layer plates were made by pouring a layer of chemotaxis agarose into tissue culture plates and then adding a thin layer of Trypticase soy agar. Neutrophils were incubated with antibiotic for 1 h and then were washed and placed in wells made in the plates. After allowing PMN to migrate under the agar toward a chemoattractant well containing formyl-methionine-leucine-phenylalanine for 3 h, Streptococcus pyogenes was streaked on top of the agar and grown overnight. PMN migration and zones of inhibition of bacterial growth were measured. Neutrophils migrated 2.51 +/- 0.16 mm toward the chemoattractant well and 1.48 +/- 0.12 mm toward the medium well; migration was not significantly affected by any of the antibiotics used. Plates with PMN incubated without antibiotic showed insignificant inhibition of bacterial growth toward chemoattractant and medium wells (0.38 +/- 0.18 and 0.14 +/- 0.12 mm, respectively; for both, P > 0.05 for difference from 0). PMN incubated with oxacillin (3 micrograms/ml), a drug not concentrated in PMN, caused a similar lack of inhibition (0.28 +/- 0.09 mm toward chemoattractant; 0.14 +/- 0.03 mm toward medium). Incubation with 30 microns of ciprofloxacin per ml resulted in inhibition that was similar in both directions (1.40 +/- 0.16 versus 1.18 +/- 0.13 mm). However, for PMN incubated with azithromycin (3 micrograms/ml), an agent highly concentrated inside phagocytes, there was a large degree of inhibition which was significantly greater in the direction of chemoattractant than in the direction of medium (3.47 +/- 0.30 versus 1.89 +/- 0.25 mm; P < 0.001), indicating that release of bioactive azithromycin by neutrophils occurred after migration. Likewise, after incubation with rifampin (10 micrograms/ml), which is also concentrated by PMN, inhibition was significantly greater in the direction of chemoattractant than in the direction of medium (1.54 +/- 0.24 versus 0.81 +/- 0.28 mm; P = 0.001). We conclude that for certain antibiotics, PMN may act as vehicles for transport and delivery of active drug to sites of infection.
Full text
PDF
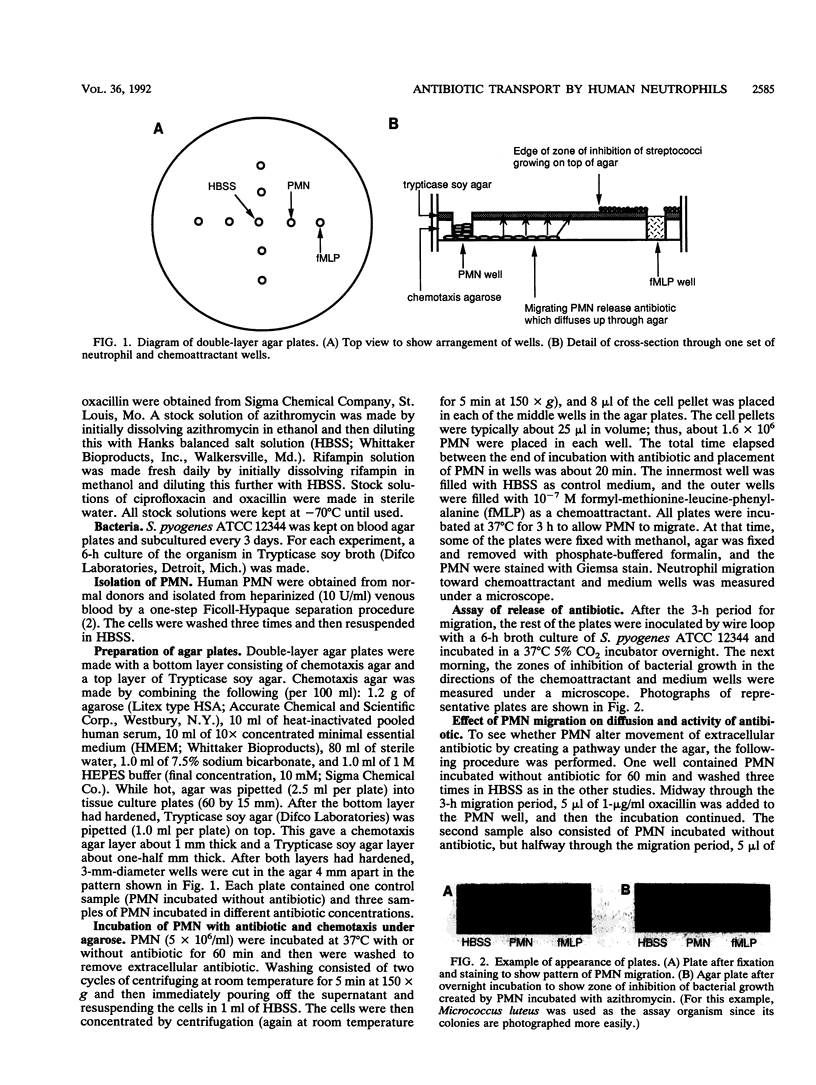

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Easmon C. S., Crane J. P. Uptake of ciprofloxacin by human neutrophils. J Antimicrob Chemother. 1985 Jul;16(1):67–73. doi: 10.1093/jac/16.1.67. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Foulds G., Shepard R. M., Johnson R. B. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- Gladue R. P., Bright G. M., Isaacson R. E., Newborg M. F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989 Mar;33(3):277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Contrasts between phagocyte antibiotic uptake and subsequent intracellular bactericidal activity. Antimicrob Agents Chemother. 1986 Jan;29(1):135–140. doi: 10.1128/aac.29.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höger P. H., Vosbeck K., Seger R., Hitzig W. H. Uptake, intracellular activity, and influence of rifampin on normal function of polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1985 Nov;28(5):667–674. doi: 10.1128/aac.28.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H. High-performance liquid chromatography measurement of antimicrobial concentrations in polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987 Dec;31(12):1904–1908. doi: 10.1128/aac.31.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGowan A. P., Holt H. A., Reeves D. S. In-vitro synergy testing of nine antimicrobial combinations against Listeria monocytogenes. J Antimicrob Chemother. 1990 Apr;25(4):561–566. doi: 10.1093/jac/25.4.561. [DOI] [PubMed] [Google Scholar]
- Prokesch R. C., Hand W. L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982 Mar;21(3):373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. J., Yin E. J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970 Apr;19(4):573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P., Matsumoto T., Husson M. Intraphagocytic penetration of antibiotics. J Antimicrob Chemother. 1988 Aug;22(2):185–192. doi: 10.1093/jac/22.2.185. [DOI] [PubMed] [Google Scholar]