Abstract
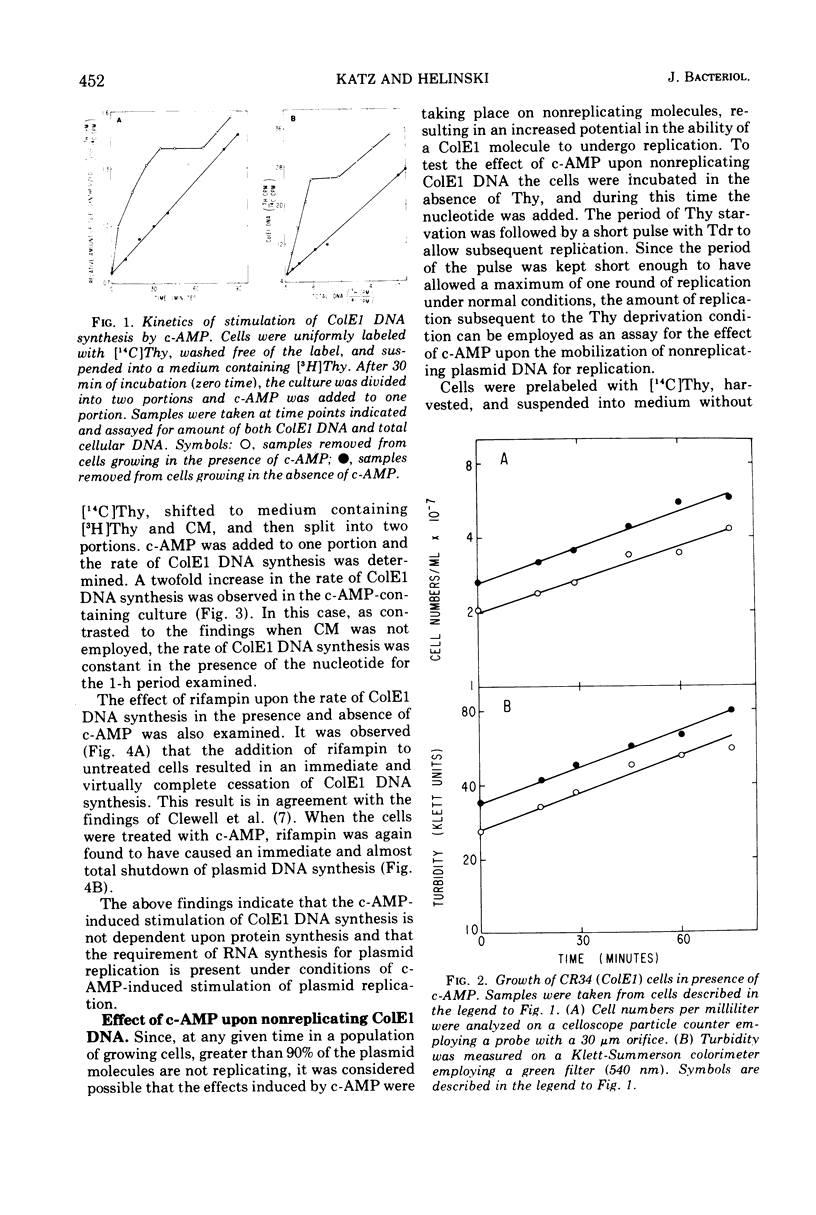
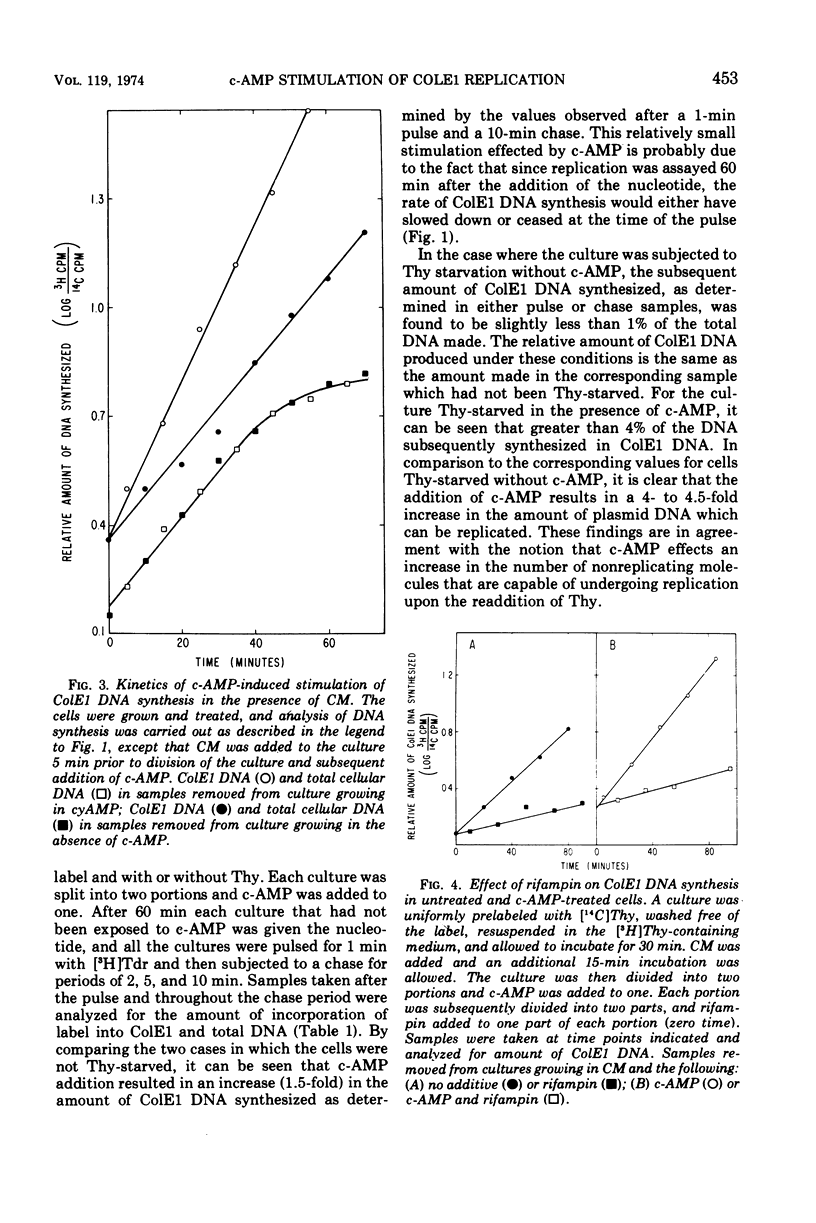
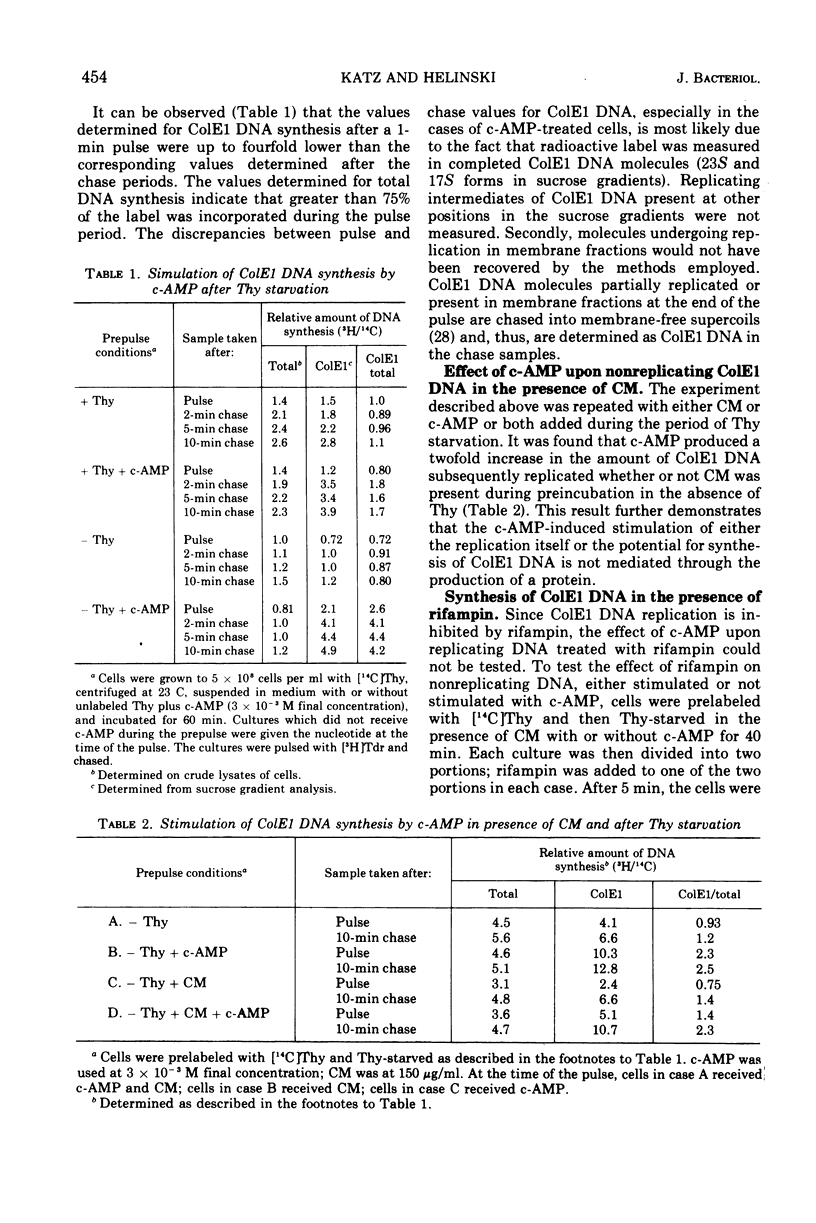
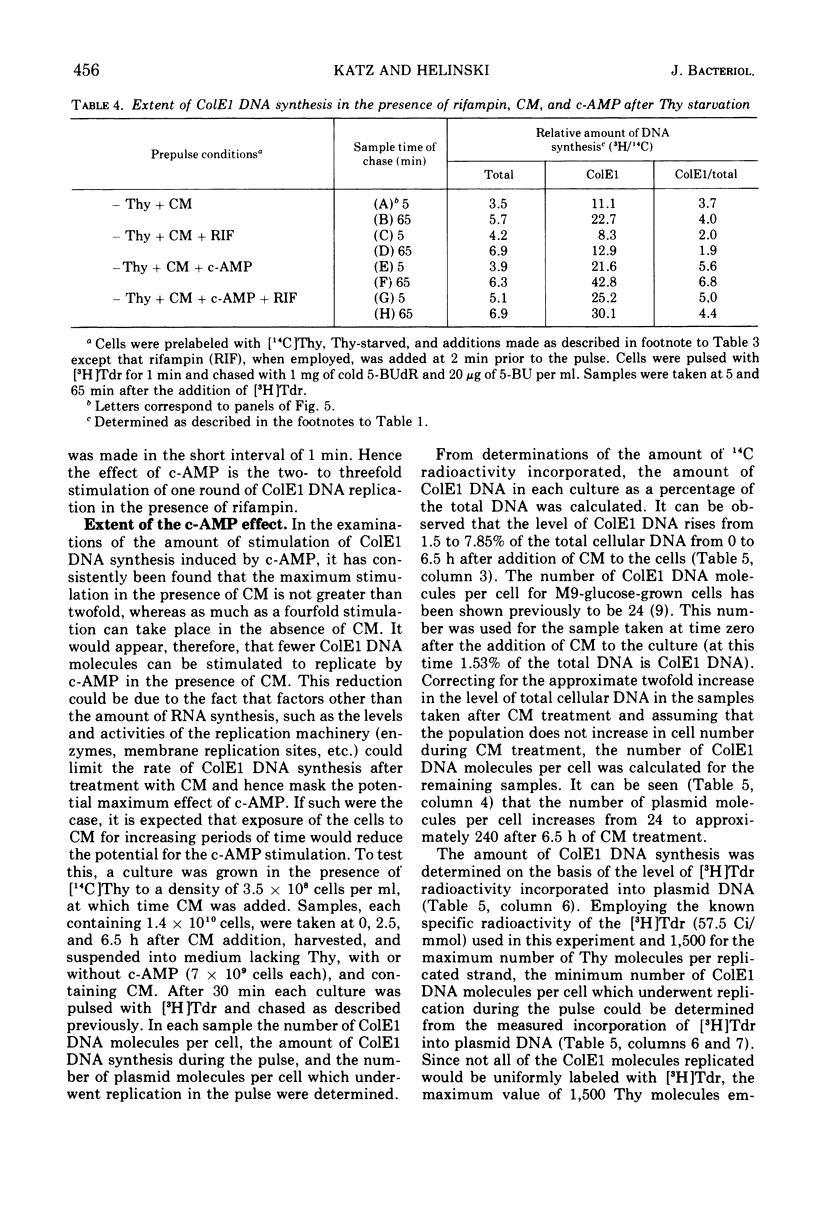
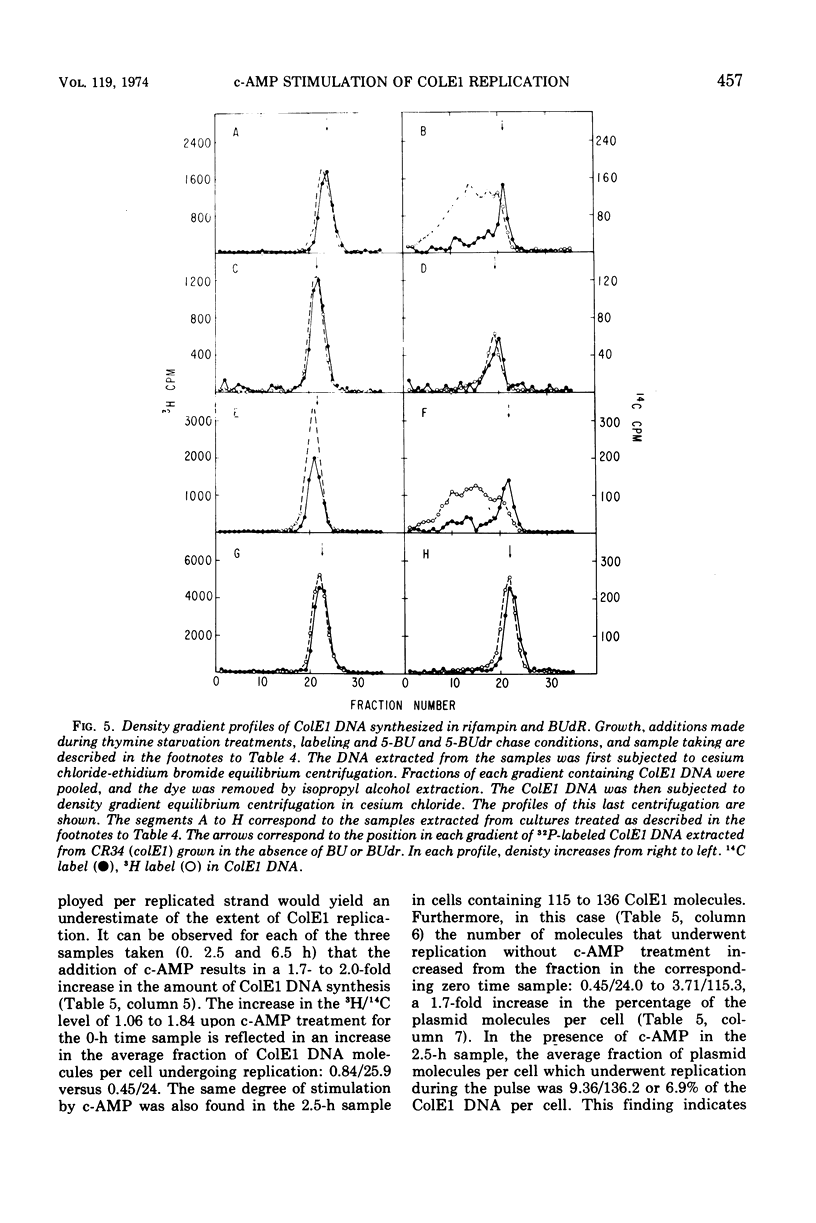
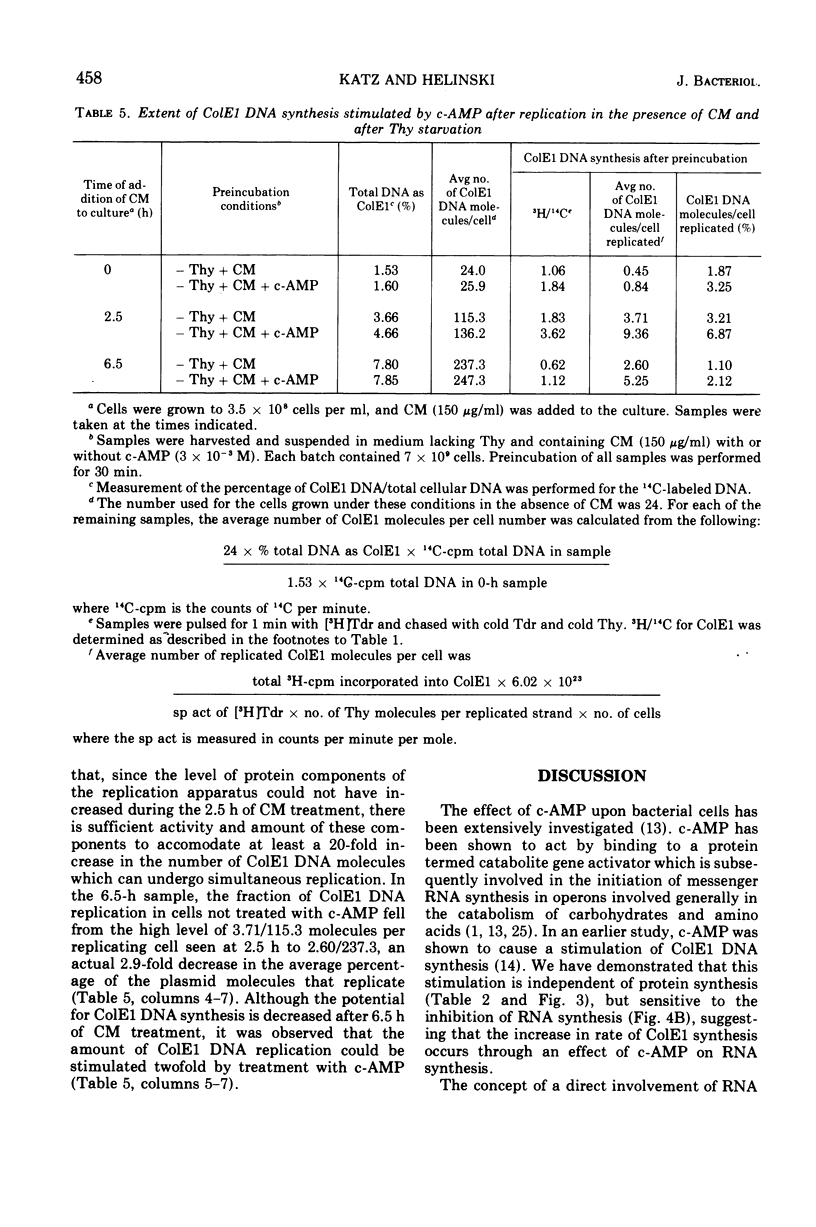
Addition of cyclic adenosine 3′-5′-monophosphate (c-AMP) to growing Escherichia coli cells, colicinogenic for the plasmid ColE1, results in a fourfold stimulation in the rate of synthesis of the plasmid deoxyribonucleic acid (DNA). The stimulation is transient (30 min) and is succeeded by a brief period (30 min) of cessation of plasmid DNA replication. The stimulation of ColE1 DNA replication also occurs in chloramphenicol-treated cells. Rifampin inhibits ColE1 DNA replication in the presence or absence of c-AMP. Employing thymine starvation conditions to stop ColE1 DNA synthesis, it was found that c-AMP, added during the period of thymine starvation, effected a stimulation in the amount of subsequent replication which took place when replicating conditions were restored. The stimulatory effect of c-AMP under these conditions was not prevented by chloramphenicol but was completely eliminated when rifampin was present. Under these conditions, when rifampin was added after the effect of c-AMP was allowed to occur, subsequent replication of the plasmid could take place, but only one round of replication occurred. A model to account for the c-AMP effects is presented.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Sherratt D. J., Clewell D. B., Helinski D. R. Isolation of supercoiled colicinogenic factor E 1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2518–2522. doi: 10.1073/pnas.69.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley P. J., Kosturko L. D., Kozinski A. W. In vivo production of an RNA-DNA copolymer after infection of Escherichia coli by bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3165–3169. doi: 10.1073/pnas.69.11.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Evenchik B. G. Effects of rifampicin, streptolydigin and actinomycin D on the replication of Col E1 plasmid DNA in Escherichia coli. J Mol Biol. 1973 Apr 15;75(3):503–513. doi: 10.1016/0022-2836(73)90457-9. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Evenchik B., Cranston J. W. Direct inhibition of Col E 1 plasmid DNA replication in Escherichia coli by rifampicin. Nat New Biol. 1972 May 3;237(70):29–31. doi: 10.1038/newbio237029a0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke M., Inselburg J. Electron microscopic studies of replicating and catenated colicin factor E1 DNA isolated from minicells (DNA replication). Proc Natl Acad Sci U S A. 1972 Jan;69(1):89–92. doi: 10.1073/pnas.69.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Isolation of catenated and replicating DNA molecules of colicin factor E1 from minicells. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2839–2842. doi: 10.1073/pnas.68.11.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli: requirement for deoxyribonucleic acid polymerase I in the replication of the plasmid ColE 1 . J Bacteriol. 1973 Jun;114(3):1116–1124. doi: 10.1128/jb.114.3.1116-1124.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C. Inhibition of plasmid DNA replication by rifampin in Salmonella pullorum. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2019–2025. doi: 10.1016/0006-291x(72)90753-x. [DOI] [PubMed] [Google Scholar]
- Kline B. C. Role of DNA transcription in the initiation of Escherichia coli sex factor (F) DNA replication. Biochem Biophys Res Commun. 1973 Jan 23;50(2):280–288. doi: 10.1016/0006-291x(73)90837-1. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Staudenbauer W. L., Hofschneider P. H. Inhibition of minicircular DNA replication in Escherichia coli 15 by rifampicin. Nat New Biol. 1972 Aug 16;238(85):202–203. doi: 10.1038/newbio238202a0. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr Assymetric annealing of an RNA linked DNA molecule isolated during the initiation of bacteriophage T7 DNA replication. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1082–1086. doi: 10.1016/0006-291x(72)90323-3. [DOI] [PubMed] [Google Scholar]
- Nakazawa A., Tamada T. Involvement of cyclic 3',5'-adenosine monophosphate in replication of colicinogenic factor E 1 DNA. Biochem Biophys Res Commun. 1972 Nov 15;49(4):977–983. doi: 10.1016/0006-291x(72)90308-7. [DOI] [PubMed] [Google Scholar]
- Nakazawa A., Tamada T. Stimulation of colicin E 1 synthesis by cyclic 3', 5'-adenosine monophosphate in mitomycin C-induced Escherichia coli. Biochem Biophys Res Commun. 1972 Jan 31;46(2):1004–1010. doi: 10.1016/s0006-291x(72)80241-9. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Reiness G., Zubay G. Purification and DNA-binding properties of the catabolite gene activator protein. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1222–1225. doi: 10.1073/pnas.68.6.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Wickner W., Westergaard O., Brutlag D., Geider K., Bertsch L. L., Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA synthesis resistant to rifampicin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2691–2695. doi: 10.1073/pnas.69.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt D. J., Helinski D. R. Replication of colicinogenic factor E1 in Escherichia coli. Properties of newly replicated supercoils. Eur J Biochem. 1973 Aug 1;37(1):95–99. doi: 10.1111/j.1432-1033.1973.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Speyer J. F., Chao J., Chao L. Ribonucleotides covalently linked to deoxyribonucleic acid in T4 bacteriophage. J Virol. 1972 Nov;10(5):902–908. doi: 10.1128/jvi.10.5.902-908.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Replication of bacteriophage M 13: inhibition of single-strand DNA synthesis by rifampicin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1634–1637. doi: 10.1073/pnas.69.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Okazaki R. RNA-linked DNA fragments in vitro. Proc Natl Acad Sci U S A. 1973 Jan;70(1):88–92. doi: 10.1073/pnas.70.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H., Boyer H. W., Helsinki D. R. Size and base composition of RNA in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3744–3748. doi: 10.1073/pnas.70.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]


