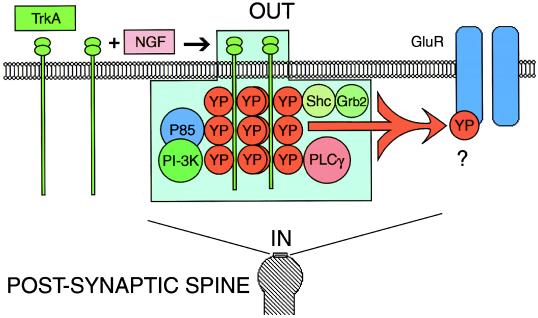
Figure 6.
Induction of tyrosine phosphorylation by NGF modulates transmission at the postsynaptic site of squid giant synapse. Activation of TrkA-like receptors in the postsynaptic site by NGF produces autophosphorylation of the receptor cytoplasmic tyrosine residues (YP). Associated downstream signaling molecules to tyrosine phosphorylated squid TrkA are still unknown. Shown are some of the molecules known to bind tyrosine-phosphorylated TrkA: phosphatidylinositol 3 kinase (PI-3K), the adaptor protein P85 (P85), phospholipase Cγ (PLCγ), and Shc (Shc) in other systems. TrkA can signal directly or indirectly, resulting in tyrosine phosphorylation (YP) and inhibition of excitatory postsynaptic potential by direct or indirect inhibition of glutamate receptors (GluR).