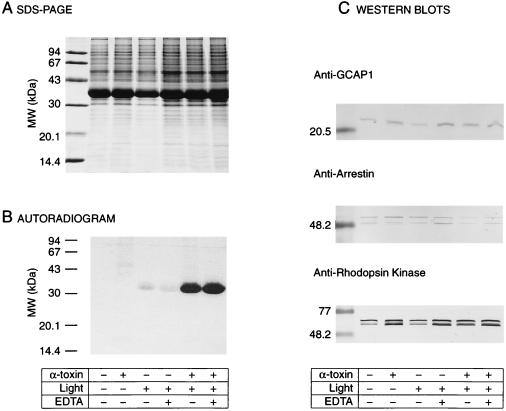
Figure 2.
Assay for Rho* phosphorylation in α-toxin-permeabilized retina. (A) SDS/PAGE. Retina punches (7.5 mm in diameter) were incubated under various experimental conditions. ROS were then purified, solubilized in 1% SDS, and a fraction (10 μl) of each sample was subjected to SDS/PAGE on a 12% gel. The amounts of Rho were comparable in all conditions. (B) Autoradiogram of the SDS/PAGE gel. Samples kept in the dark showed no phosphorylation in the presence or absence of α-toxin (lanes 1 and 2). In samples exposed to light, an increase in radiolabeled phosphorylation of Rho* in retina permeabilized with α-toxin (100 μg/ml) is clearly visible (lanes 5 and 6) as compared with nonpermeabilized retina (lanes 3 and 4). EDTA (4 mM) was added to the incubation buffer (lanes 4 and 6) as a control for nonspecific phosphorylation or phosphorylation resulting from broken ROS. (C) Western blots. ROS samples were immunoblotted and labeled with antibodies against several ROS proteins (GCAP1, arrestin, and rhodopsin kinase). α-Toxin is required for the light-dependent phosphorylation, and permeabilized retina retains protein >20 kDa.