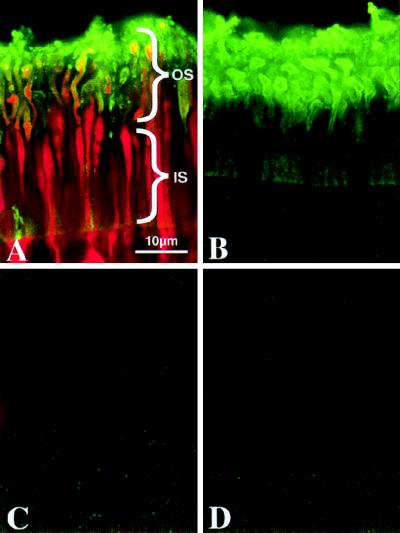
Figure 3.
Permeability of rod plasma membranes containing the Zn2+-sensitive form of the α-toxin pore (H5K8A) demonstrated by confocal fluorescence localization of low molecular weight intracellular tracer. Retinal punches were incubated in the presence (A and B) or absence (C and D) of α-toxin. The addition of Zn2+ to the incubation medium causes pore closure (B and D). The low molecular weight intracellular tracer N-(2-aminoethyl) biotinamide hydrochloride (Neurobiotin) was added to all samples to assess permeability of α-toxin pores under various conditions. An α-toxin-specific polyclonal antibody was used to immunolocalize this protein (green). α-Toxin is restricted to the plasma membrane-surrounding rod inner segments (IS) and outer segments (OS) of treated retinas (A and B). Streptavidin-Cy3 was used to localize neurobiotin in these samples (red). Neurobiotin is present in the cytoplasm of rod OS and IS (A), confirming that α-toxin forms functional pores in these cells. Neurobiotin is prevented from diffusing through α-toxin pores closed by Zn2+ (B).