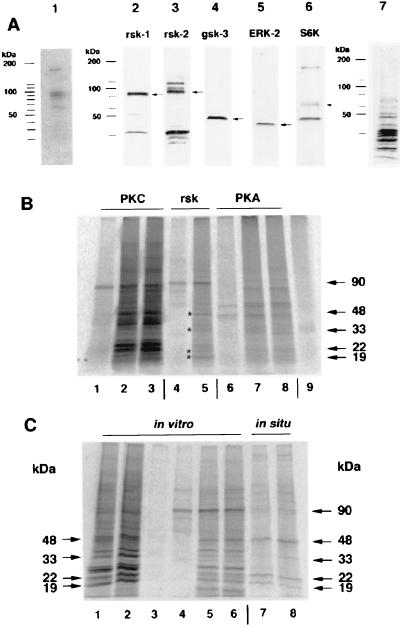
Figure 3.
A group of kinases is bound to polyribosomal complexes prepared from synaptoneurosomes. (A) Detection of autophosphorylation activity in kinases from the 0.5 M K+ fraction. Lane 1, PRBPs (25 μg) were prepared as described (15, 16) and after electrophoretic separation blotted to a poly(vinylidene difluoride) membrane. The proteins were renatured, and the presence of kinases was detected by autophosphorylation in the presence of [γ-33P]-ATP according to the method of Ferrell (27). This procedure revealed five kinases with molecular masses of about 65, 85, 90, 95, and 180 kDa. Identification of different kinases among PRBP by Western blot assay. Lane 2, p90rsk-1. Lane 3, p90rsk-2, the antiserum to p90rsk-2 crossreacts with two unknown additional proteins with molecular masses of about 100 and 115 kDa. Lane 4, gsk-3β. Lane 5, ribosomal S6 kinase p70S6K. Lane 6, ERK-2 (p42MAPK). Lane 7, an amido black protein staining of PRBPs on nitrocellulose. (B) In vitro phosphorylation of PRBPs by added kinases after blocking the activity of all endogenous kinases by heating the fraction to 70°C for 5 min. Lane 1, autophosphorylated form of PKC alone; lane 2, PRBP and PKC without activators; lane 3, PRBP and PKC with activators; lane 4, autophosphorylated p90rsk-2; lane 5, PRBP and p90rsk-2 (* indicates the position of proteins that are also phosphorylated in situ); lane 6, autophosphorylated PKA; lane 7, PRBP and PKA; lane 8, PRBP, PKA, and cAMP; lane 9, PRBP. Note that the specific activities of the kinases are not equal, because p90rsk-2 is only partially purified. (C) Phosphorylation pattern of PRBP under different conditions. Lanes 1–2, all polyribosome-bound kinases (lane 1 with 10 μM GF109203X and lane 2 without inhibitor). Lanes 3–6, effect of p90rsk-2 (lane 3, heated PRBP; lane 4, p90rsk-2 alone, lane 5 and 6 p90rsk-2 with heated PRBP). Lanes 7–8, phosphorylation pattern of PRBP under in situ conditions. All endogenous phosphorylated proteins (arrows on right) are in vitro substrates for p90rsk-2. The 85- and 90-kDa protein phosphorylated under in situ conditions might be p90rsk-1 and p90rsk-2 itself.