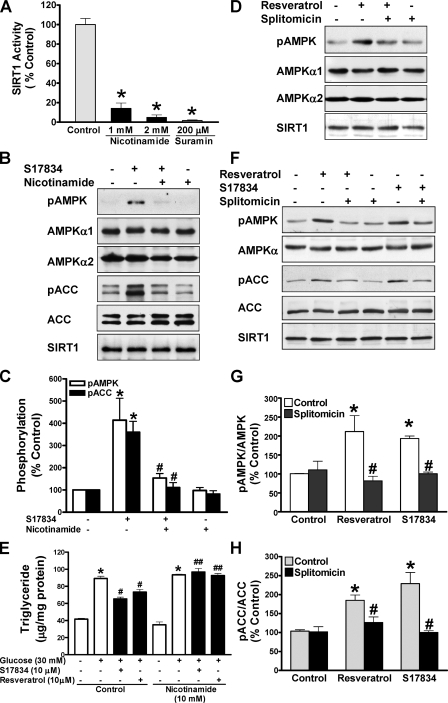
FIGURE 2.
Pharmacological inhibition of SIRT1 attenuates polyphenol-induced AMPK activation and lipid reduction in human HepG2 cells or HEK293 cells. A, SIRT1 deacetylase activity is largely inhibited by nicotinamide. The SIRT1 activity assay was carried out in the absence or presence of nicotinamide or suramin, an SIRT1 inhibitor included in the SIRT1 activity assay kit. *, p < 0.05 versus control. B–D, inhibition of SIRT1 activity by nicotinamide or splitomicin diminishes enhanced phosphorylation of AMPK and ACC in response to the polyphenol in HepG2 cells. Cells were pretreated without or with either nicotinamide (10 mm) or splitomicin (100 μm) in the serum-free medium for 24 h and then incubated without or with S17834 (10 μm) or resveratrol (50 μm) for an another 1 h. Densitometric quantification of the phosphorylation of AMPK and ACC is shown. *, p < 0.05 versus control; #, p < 0.05 versus polyphenol alone (mean ± S.E., n = 3). E, nicotinamide prevents the lipid-lowering effect of polyphenols in HepG2 cells. Cells were pretreated for 24 h without or with nicotinamide (10 mm) in serum-free medium and incubated for 24 h without or with polyphenols in the presence of high glucose. *, p < 0.05 versus normal glucose alone; #, p < 0.05 versus high glucose alone; ##, p < 0.05 versus high glucose plus polyphenols (mean ± S.E., n = 4). F–H, the polyphenols increase and splitomicin decreases phosphorylation of AMPK and ACC in HEK293 cells. Phosphorylation of AMPK and ACC was assessed in cells pretreated with splitomicin (100μm) for 24 h and incubated with resveratrol (50 μm) or S17834 (10 μm) for an additional 1 h as indicated. *, p < 0.05 versus control; #, p < 0.05 versus polyphenol alone (mean ± S.E., n = 3). No detectable change in the expression of endogenous SIRT1 was observed throughout treatments in both human cell lines.