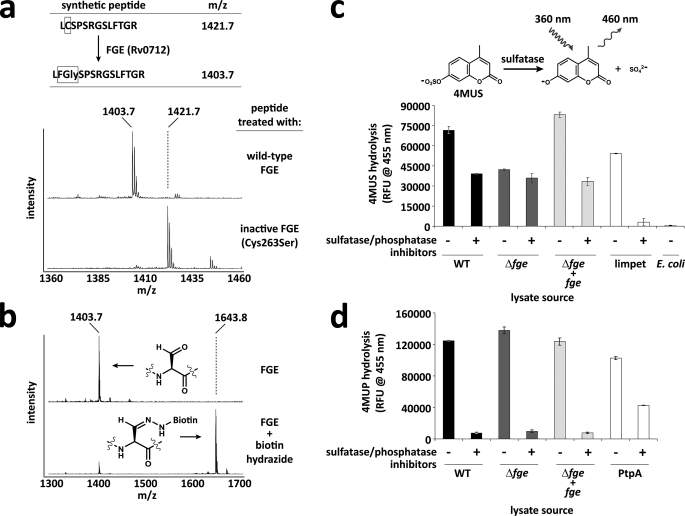
FIGURE 2.
Function of M. tuberculosis FGE (Rv0712) in vitro and in vivo. a, a synthetic peptide resembling a sulfatase motif was treated with recombinant M. tuberculosis FGE, and the resulting oxidation of cysteine to FGly was monitored by mass spectrometry. The C263S FGE mutant was inactive on the peptide substrate. Theionsatm/z 1427 and 1445 are sodium adducts of the modified and unmodified peptide, respectively. b, upon treatment with biotin hydrazide, the FGly-containing peptide forms a hydrazone adduct with biotin, resulting in a mass shift of +240 Da. c, lysates from wild-type (WT), Δfge, and complemented (Δfge + fge) strains of M. tuberculosis H37Rv were tested for sulfatase activity using the fluorogenic substrate 4-methylumbelliferyl sulfate (4MUS) with and without sulfatase/phosphatase inhibitors. Limpet sulfatase was used as a positive control. E. coli lysate was used as a negative control. d, lysates from WT, Δfge, and Δfge + fge strains of M. tuberculosis H37Rv were tested for phosphatase activity using the fluorogenic substrate 4-methylumbelliferyl phosphate with and without sulfatase/phosphatase inhibitors. The recombinant M. tuberculosis phosphatase PtpA was used as a positive control. Error bars represent S.D. ± mean between three independent experiments.