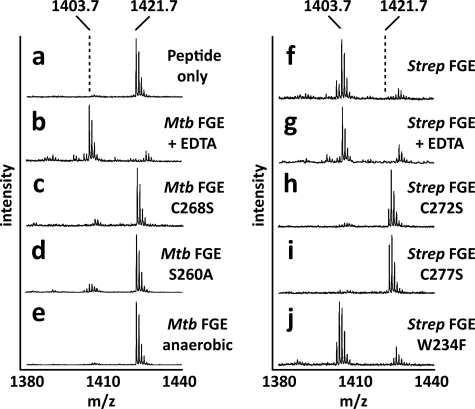
FIGURE 4.
M. tuberculosis and S. coelicolor FGE activity is dependent upon molecular oxygen but independent of metal cofactors. a, a synthetic peptide resembling the sulfatase motif (mass, 1421.7 Da) was used as a substrate for FGE. Conversion of cysteine to FGly resulted in a loss of 18 Da that was detected by mass spectrometry. b and g, metal chelator EDTA had no effect on activity. c, h, i, loss of active site cysteines in M. tuberculosis and S. coelicolor FGE abolished activity. d, loss of active site Ser-260 in M. tuberculosis FGE significantly reduced activity. e, M. tuberculosis FGE was inactive in the absence of molecular oxygen. f, WT S. coelicolor FGE was able to oxidize the synthetic peptide. j, active site Trp-234 in S. coelicolor FGE is not essential for catalytic activity. The ion at m/z 1427 is the sodium adduct of the FGly-containing product peptide. Mtb, M. tuberculosis; Strep, S. coelicolor.