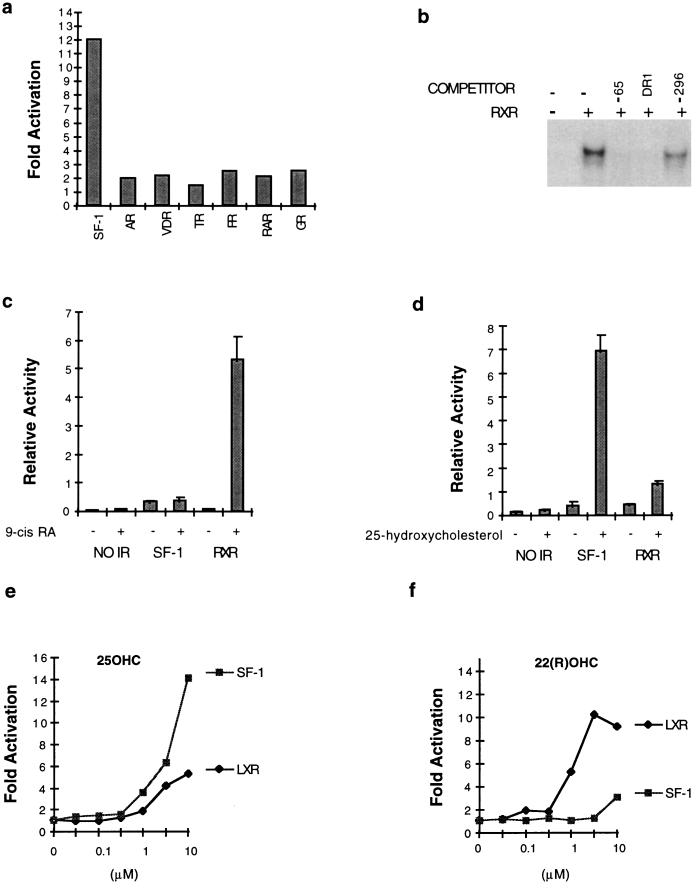
Figure 4.
(a) SF-1 is preferentially activated by 25OHC. The indicated nuclear receptors were analyzed in the cotransfection assay with 25OHC. All receptors were tested on their respective response elements, and results are given as fold-activation relative to cells without 25OHC. AR, androgen receptor; TR, thyroid hormone receptor; RAR, RA receptor; PR, progesterone receptor; VDR, vitamin D receptor; and GR, glucocorticoid receptor. (b) RXR specifically binds the 21-hydroxylase −65 element. One hundred × excess of unlabeled −65 oligos (1) and a classical RXR binding site (DR-1) abolish RXR binding whereas another SF-1 binding site (−296) (1) is a weaker competitor. (c) RXR responds to 9-cis (1 μM) whereas SF-1 does not. (d) SF-1 activity is enhanced in response to 25OHC (10 μM) whereas RXR responds weakly. (e) 25OHC is a strong activator of SF-1 but weak for LXR. (f) Conversely, 22(R)OHC is a strong activator of LXR but weak for SF-1.