Abstract
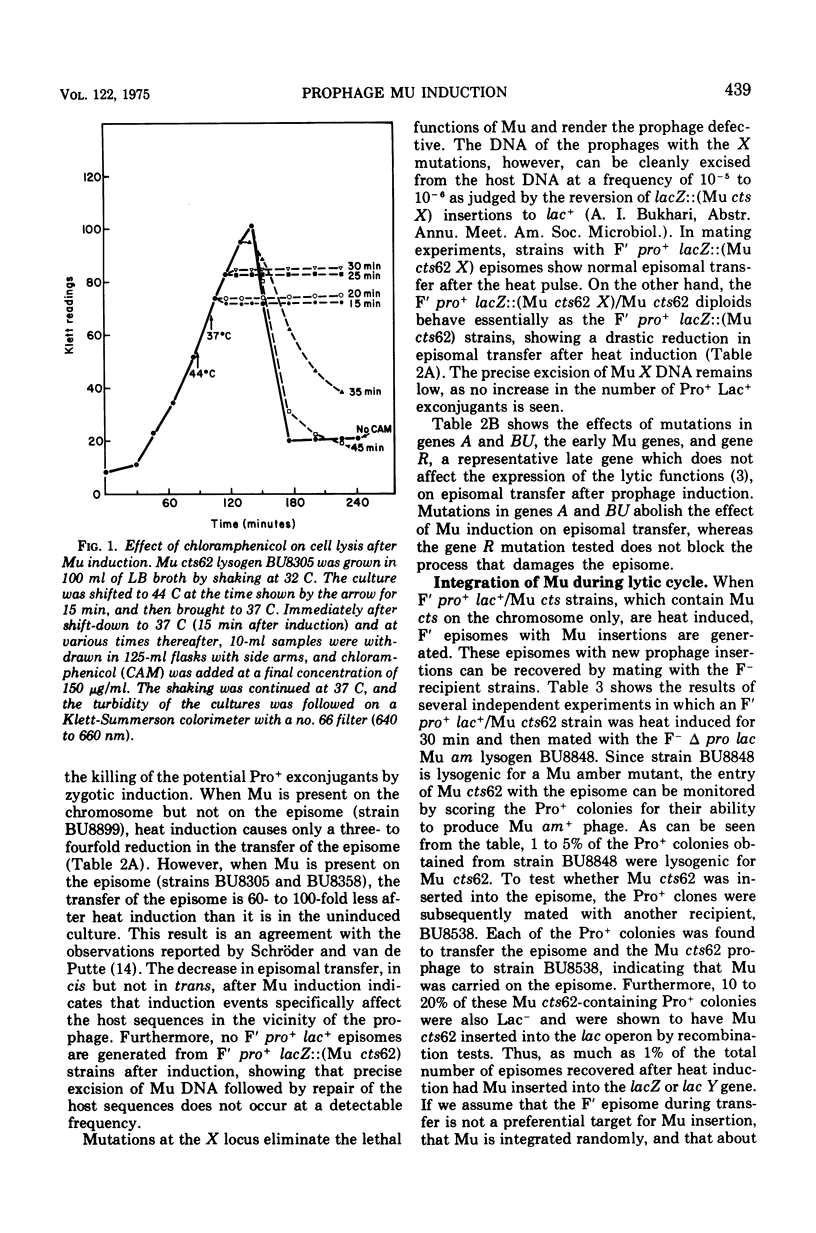
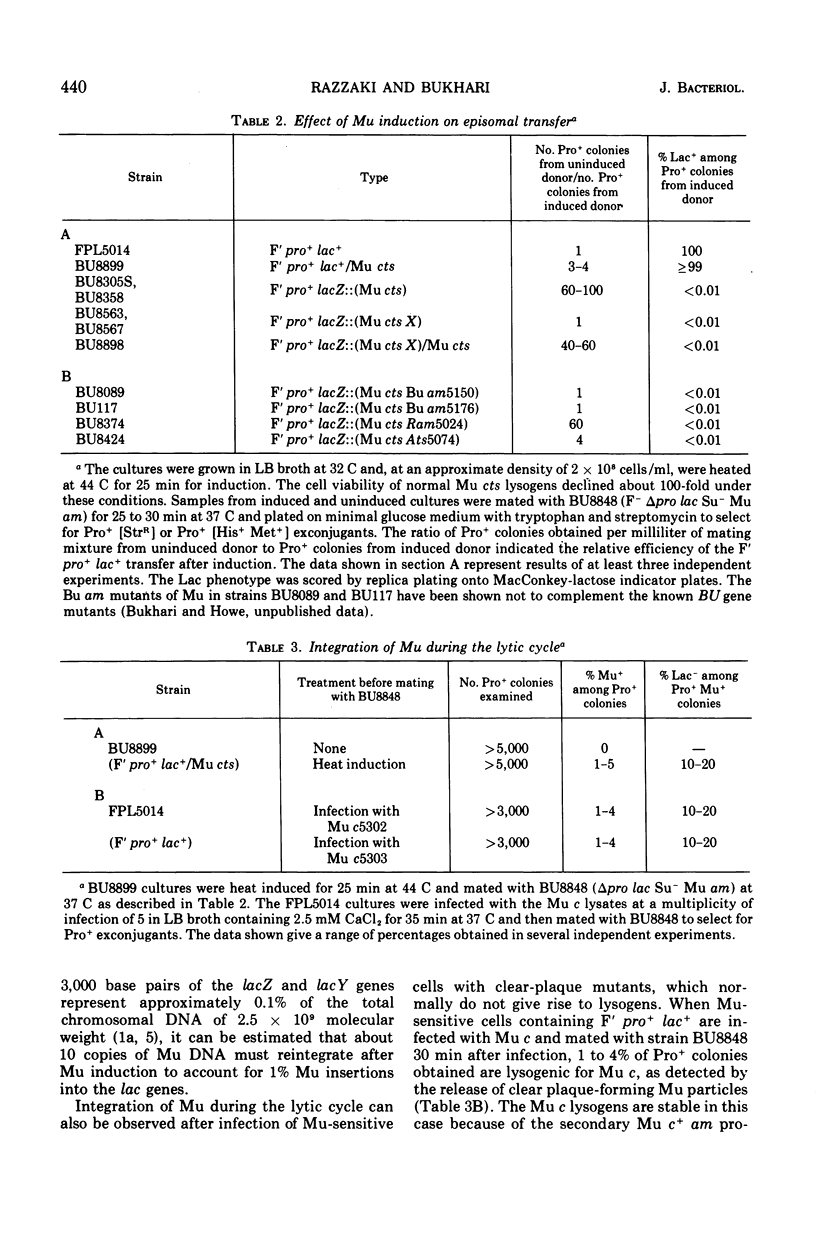
Escherichia coli strains lysogenic for a thermoinducible Mu prophage (Mu cts62) undergo rapid lysis about 50 min after heat induction. Induction of Mu cts62 apparently causes damage to the host sequences in which Mu is inserted. The normal expression of A, BU, and X genes of Mu is needed for this specific deleterious effect on the prophage-containing host sequences. Mu deoxyribonucleic acid can be shown to reintegrate extensively at different sites on the host genome during the lytic cycle after prophage induction or after infection of sensitive cells by clear-plaque mutants of Mu. We estimate that approximately 10 copies of Mu deoxyribonucleic acid are inserted per chromosome during vegetative growth. The episome rescue method for detecting vegetative Mu deoxyribonucleic acid insertion, in which an episome is transferred from the lytically infected cells to F- receipient cells, can be applied to study Mu integration without requiring the host cells to survive. It also provides an easy system to isolate Mu insertions in transmissible episomes and plasmids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J., Boram W., Bukhari A. I., Faelen M., Howe M., Metlay M., Taylor A. L., Toussaint A., Van de Putte P., Westmaas G. C. Summary of the genetic mapping of prophage Mu. Virology. 1973 Jul;54(1):90–92. doi: 10.1016/0042-6822(73)90117-7. [DOI] [PubMed] [Google Scholar]
- Boram W., Abelson J. Bacteriophage Mu integration: on the mechanism of Mu-induced mutations. J Mol Biol. 1971 Nov 28;62(1):171–178. doi: 10.1016/0022-2836(71)90137-9. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Allet B. Plaque-forming lambda-Mu hybrids. Virology. 1975 Jan;63(1):30–39. doi: 10.1016/0042-6822(75)90367-0. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Curtin P. Temperature-sensitive mutants of bacteriophage mu. J Virol. 1974 Dec;14(6):1615–1616. doi: 10.1128/jvi.14.6.1615-1616.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Metlay M. Genetic mapping of prophage Mu. Virology. 1973 Jul;54(1):109–116. doi: 10.1016/0042-6822(73)90120-7. [DOI] [PubMed] [Google Scholar]
- Daniell E., Abelson J., Kim J. S., Davidson N. Heteroduplex structures of bacteriophage Mu DNA. Virology. 1973 Jan;51(1):237–239. doi: 10.1016/0042-6822(73)90385-1. [DOI] [PubMed] [Google Scholar]
- Daniell E., Roberts R., Abelson J. Mutations in the lactose operon caused by bacteriophage Mu. J Mol Biol. 1972 Aug 14;69(1):1–8. doi: 10.1016/0022-2836(72)90019-8. [DOI] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Control of gene expression in bacteriophage lambda. Annu Rev Genet. 1973;7:289–324. doi: 10.1146/annurev.ge.07.120173.001445. [DOI] [PubMed] [Google Scholar]
- Howe M. M. Prophage deletion mapping of bacteriophage Mu-1. Virology. 1973 Jul;54(1):93–101. doi: 10.1016/0042-6822(73)90118-9. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuscelli J., Taylor A. L., Cummings D. J., Chapman V. A., DeLong S. S., Cañedo L. Electron microscopic evidence for linear insertion of bacteriophage MU-1 in lysogenic bacteria. J Virol. 1971 Oct;8(4):551–563. doi: 10.1128/jvi.8.4.551-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W., Bade E. G., Delius H. Participation of Escherichia coli DNA in the replication of temperate bacteriophage Mu1. Virology. 1974 Aug;60(2):534–542. doi: 10.1016/0042-6822(74)90347-x. [DOI] [PubMed] [Google Scholar]
- Schröder W., van de Putte P. Genetic study of prophage excision with a temperature inducible mutant of Mu-1. Mol Gen Genet. 1974 May 21;130(2):99–104. doi: 10.1007/BF00269081. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner B. T., González N. S., Taylor A. L. Isolation of heterogeneous circular DNA from induced lysogens of bacteriophage Mu-1. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1255–1259. doi: 10.1073/pnas.71.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijffelman C., Gassler M., Stevens W. F., van de Putte P. On the control of transcription of bacteriophage Mu. Mol Gen Genet. 1974;131(2):85–96. doi: 10.1007/BF00266145. [DOI] [PubMed] [Google Scholar]



