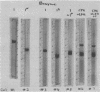
Abstract
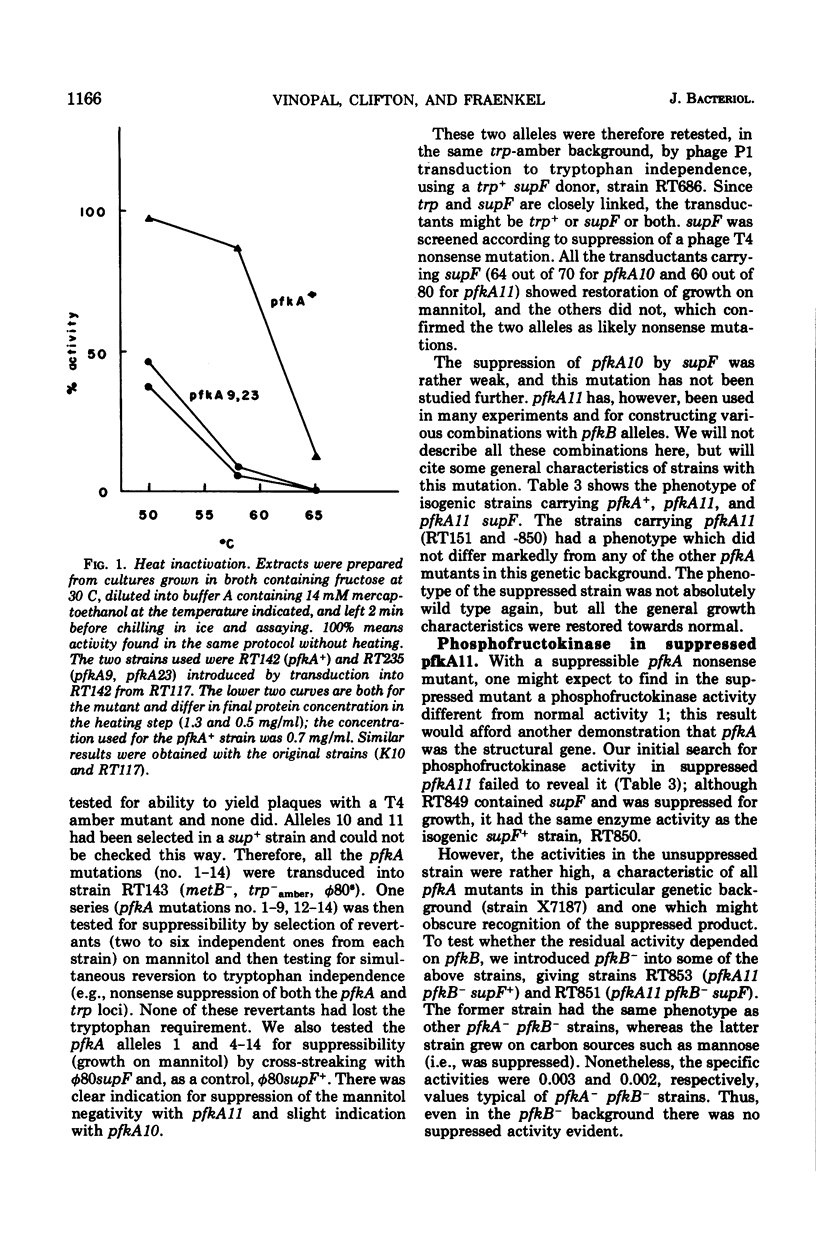
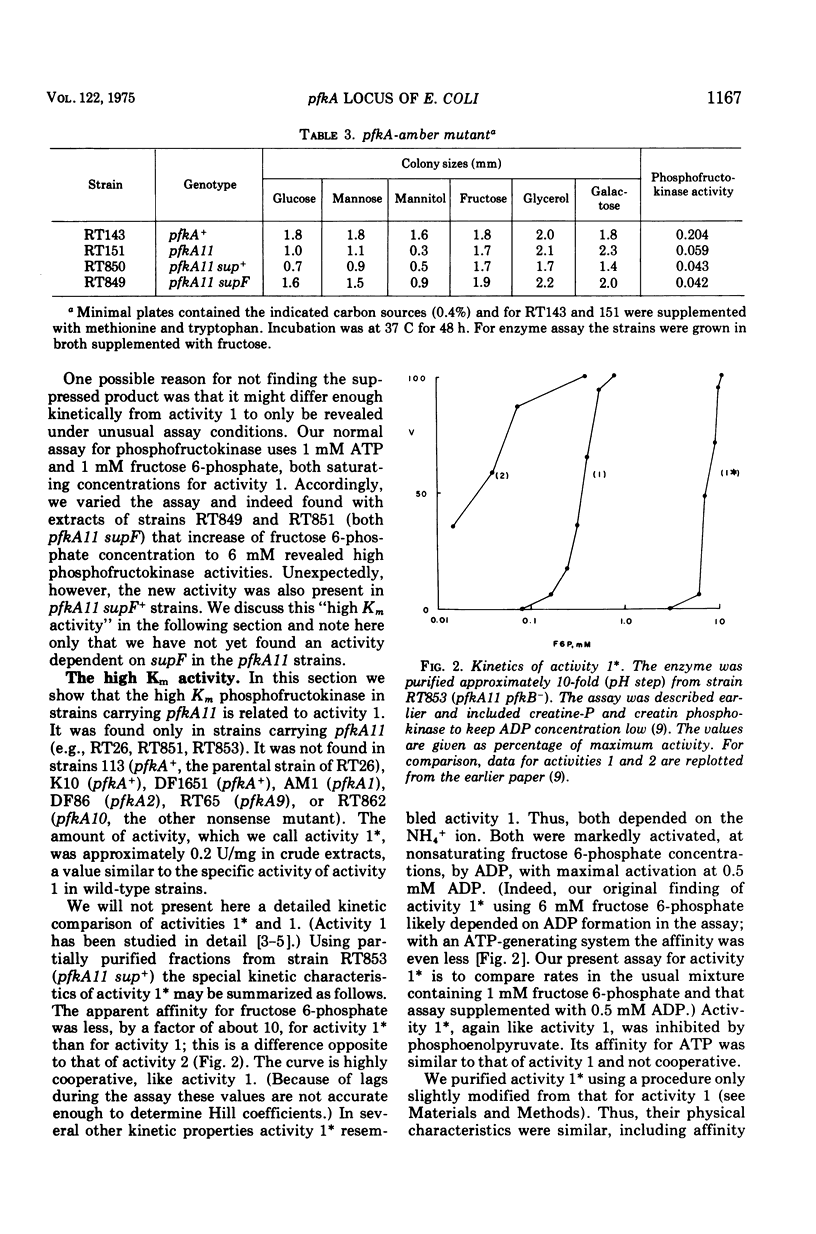
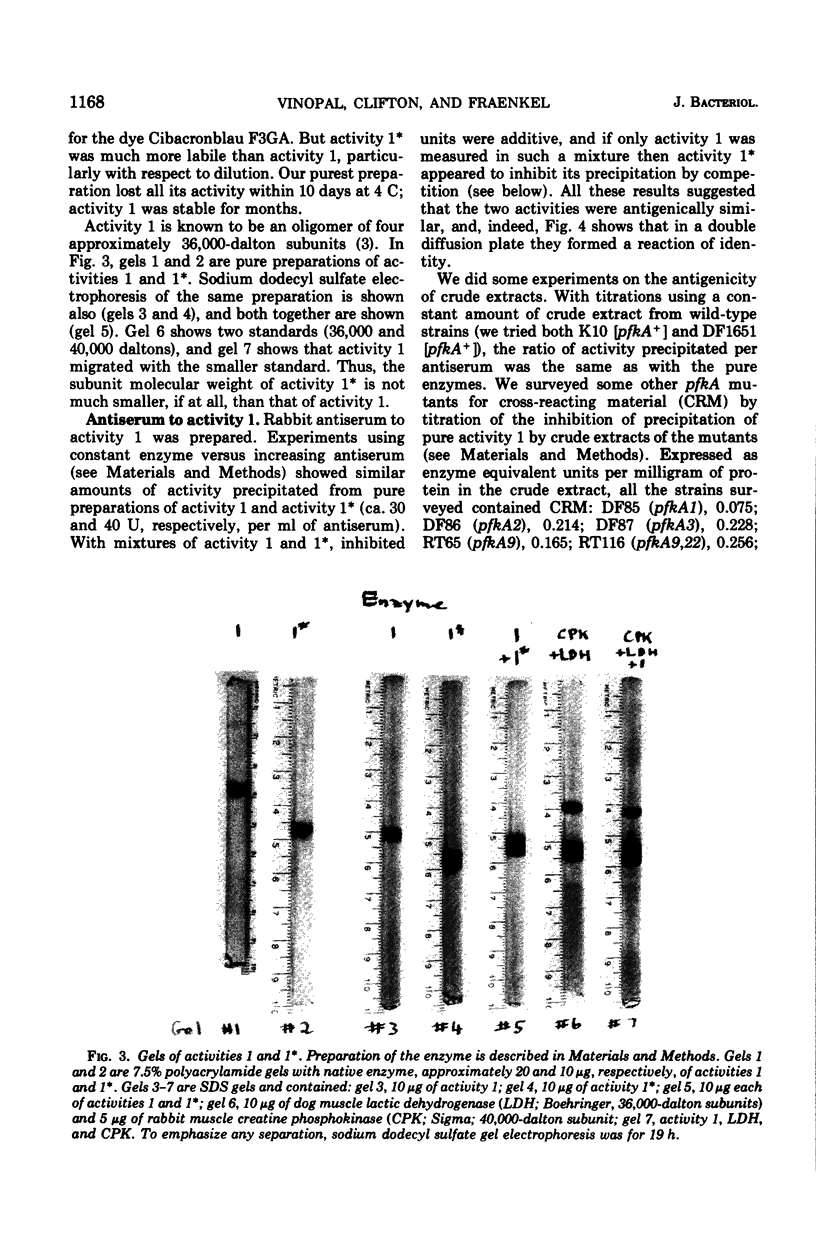
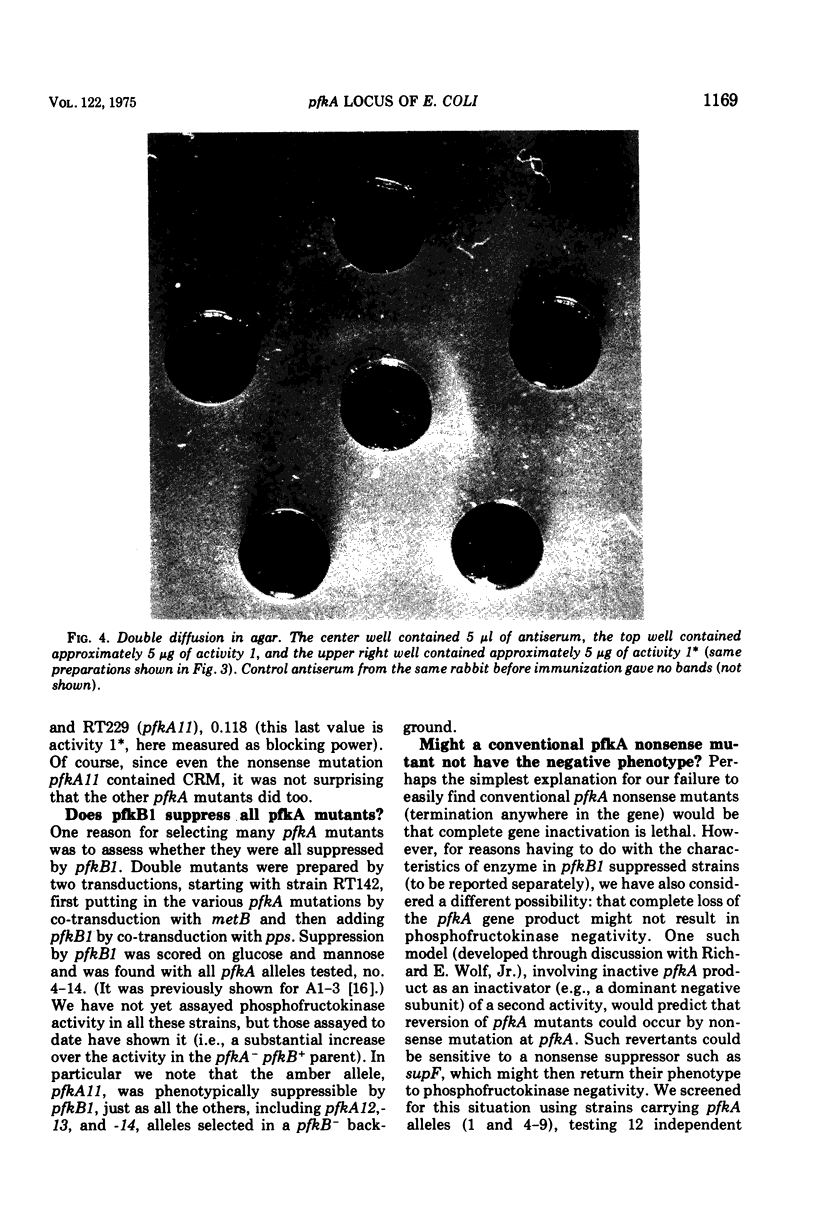
pfkA was know, on the basis of three mutants, as the likely locus of phosphofructokinase in Escherichia coli, and the unlinked pfkB1 mutation suppressed these mutations by restoring some enzyme activity (Morrissey and Fraenkel, 1972). We now report a new search for the complete inactivation of pfkA (e.g., by deletion or amber mutation), done to assess whether the pfkB1 suppression is by an independent enzyme, phosphofructokinase activity 2 (Fraenkel, Kotlarz, and Buc, 1973). Ten new phosphofructokinase mutants all were at pfkA, rather than at pfkB or pfkC. One of them (pfkA9) gave temperature-sensitive reverants with heat-labile enzyme. Another (pfkA11) proved genetically to be a nonsense mutation, but showed no restored activity when suppressed by supF. However, even unsuppressed it was found to contain an enzyme related to phosphofructokinase activity 1 kinetically (more allosteric), physically (almot identical subunit), and antigenically. All the pfkA mutants apparently contained cross-reacting material to activity 1. All (including pfkA11) were suppressed by the pfkB1 mutation. Several results support the idea that pfkA is the structural gene for the main phosphofructokinase of E. coli (activity 1), but that there is some restriction to its complete inactivation.
Full text
PDF

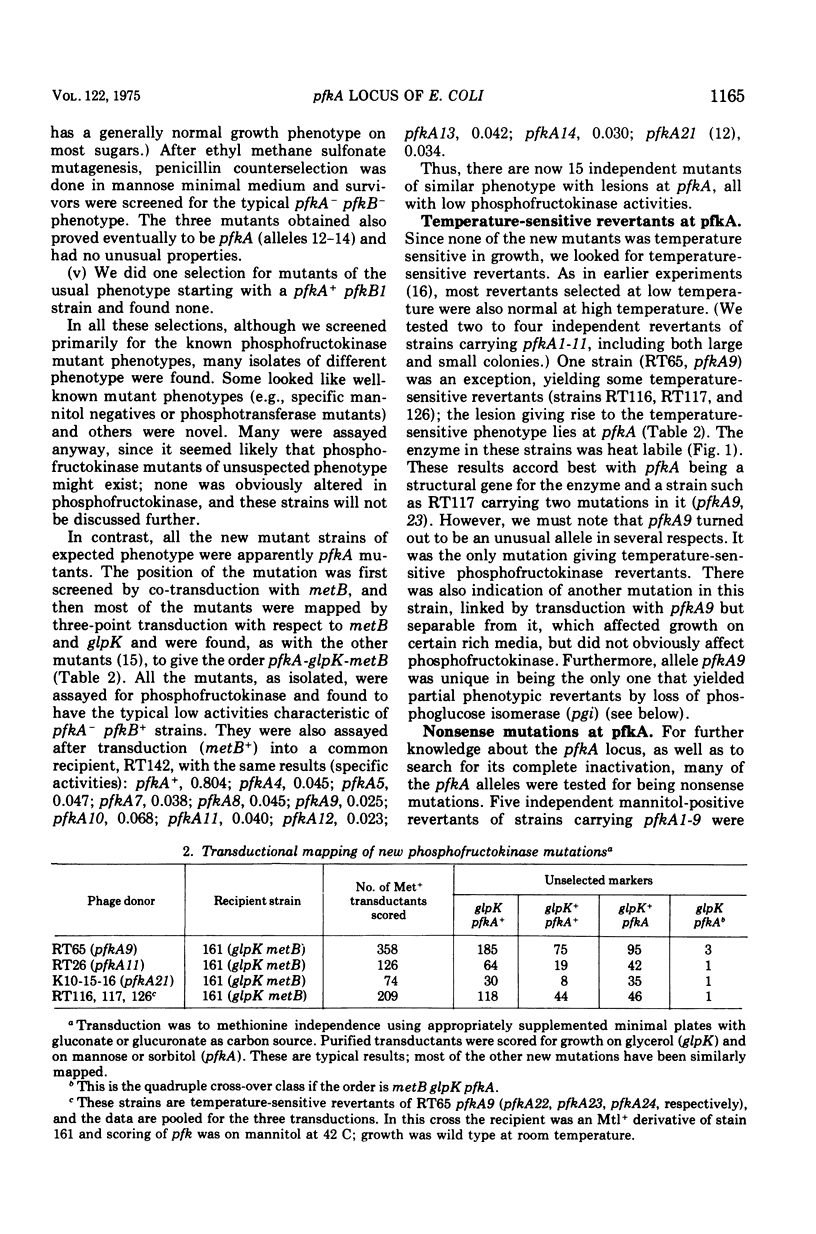


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A., Cooper R. A. Genetic mapping of a locus for triosephosphate isomerase on the genome of Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):329–334. doi: 10.1099/00221287-62-3-329. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy D., Buc H., Monod J. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J Mol Biol. 1968 Jan 14;31(1):13–35. doi: 10.1016/0022-2836(68)90051-x. [DOI] [PubMed] [Google Scholar]
- Blangy D. Phosphofructokinase from E. Coli: Evidence for a tetrameric structure of the enzyme. FEBS Lett. 1968 Dec;2(2):109–111. doi: 10.1016/0014-5793(68)80115-2. [DOI] [PubMed] [Google Scholar]
- Blangy D. Propriétés allostériques de la phosphofructokinase d'E. coli. Etude de la fixation des ligands par dialyse à l'équilibre. Biochimie. 1971;53(2):135–144. doi: 10.1016/s0300-9084(71)80044-5. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G., Kotlarz D., Buc H. Two fructose 6-phosphate kinase activities in Escherichia coli. J Biol Chem. 1973 Jul 10;248(13):4865–4866. [PubMed] [Google Scholar]
- Gottesman S., Beckwith J. R. Directed transposition of the arabinose operon: a technique for the isolation of specialized transducing bacteriophages for any Escherichia coli gene. J Mol Biol. 1969 Aug 28;44(1):117–127. doi: 10.1016/0022-2836(69)90408-2. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Role of phosphofructokinase in the utilization of glucose by Escherichia coli. Nature. 1970 Jul 4;227(5253):44–46. doi: 10.1038/227044a0. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Chromosomal location of a gene for fructose 6-phosphate kinase in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1108–1109. doi: 10.1128/jb.100.2.1108-1109.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Selection of fructose 6-phosphate kinase mutants in Escherichia coli. Biochem Biophys Res Commun. 1968 Aug 13;32(3):467–473. doi: 10.1016/0006-291x(68)90685-2. [DOI] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Suppressor of phosphofructokinase mutations of Escherichia coli. J Bacteriol. 1972 Oct;112(1):183–187. doi: 10.1128/jb.112.1.183-187.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol. 1974 Feb;117(2):641–651. doi: 10.1128/jb.117.2.641-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Lagarde A. Système de transport du 2-céto-3-désoxy-gluconate chez E. coli K 12: localisation d'un gène de structure et de son opérateur. Mol Gen Genet. 1973 Mar 1;121(2):163–180. doi: 10.1007/BF00277530. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Wilding P., Haverback B. J. A new method for the determination of alpha-amylase. Experientia. 1967 Oct 15;23(10):805–805. doi: 10.1007/BF02146851. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Fraenkel D. G. PfkB and pfkC loci of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1153–1161. doi: 10.1128/jb.122.3.1153-1161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Fraenkel D. G. Phenotypic suppression of phosphofructokinase mutations in Escherichia coli by constitutive expression of the glyoxylate shunt. J Bacteriol. 1974 Jun;118(3):1090–1100. doi: 10.1128/jb.118.3.1090-1100.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Hillman J. D., Schulman H., Reznikoff W. S., Fraenkel D. G. New phosphoglucose isomerase mutants of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1172–1174. doi: 10.1128/jb.122.3.1172-1174.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]