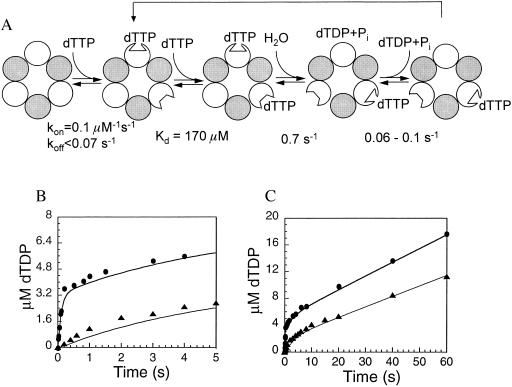
Figure 4.
Kinetic simulation of the sequential dTTP hydrolysis mechanism of 4A′. (A) Proposed mechanism of dTTP hydrolysis by 4A′ hexamer. (B and C) The acid-quench (▴) and pulse–chase (•) kinetics from Figs. 1 and 2 in different time windows. The solid lines in B and C are simulated curves created by hopkinsim using the mechanism and the rate constants shown in A. The sequential binding and hydrolysis of two dTTP and the conformational change that leads to tight binding of second dTTP were simulated as follows. The first dTTP binding was assumed to be tight and the second dTTP binding was assumed to be a rapid equilibrium step. Thus, the first dTTP in the E⋅(TTP)2 species was fully chased to product but the second dTTP was not chased to product in the pulse–chase experiment. Because the second dTTP becomes tightly bound after hydrolysis of the first dTTP, the dTTP in the E⋅TDP⋅Pi⋅TTP species was chased to product in the chase experiment.