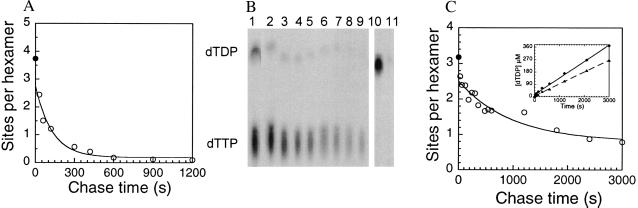
Figure 5.
Identification of 4A′-bound nucleotides and their dissociation kinetics. (A) 4A′ (2 μM hexamer) and [α-32P]dTTP (200 μM) were mixed at 18°C for 30 s and nonradiolabeled dTTP (10 mM) was added at time zero. After varying chase times (30 s to 20 min), aliquots were filtered through NC membranes. A total of 3.7 ± 0.87 nt (error calculated from seven independent measurements) were bound to 4A′ at zero chase time (•). After addition of dTTP chase, the 2–3 4A′-bound nucleotides exchanged at 0.008 ± 0.002 s−1 (○). (B) To identity the tightly bound nucleotides, NC membranes were extracted and the eluted nucleotides were analyzed by polyethyleneimine-cellulose TLC. Here the PhosphorImager scan of the TLC plate is shown. Lane 1, dTDP (20%) and dTTP (80%) bound to 4A′ before chase was added. Lanes 2–9, nucleotides bound after 0.5, 3, 6, 9, 12, 15, 20, 25 min of chase. In all lanes, dTDP is 6–17% and dTTP is 94–83%. Lane 10, dTDP bound to 4A′ after 90 min of incubation. Lane 11, almost no dTDP remained bound after 30-s chase with unlabeled dTTP. (C) The exchange of 4A′-bound dTMP-PCP was measured by preincubating 4A′ (0.83 μM hexamer) with [α-32P]dTMP-PCP (98 μM) and adding 5 mM dTTP as chase. Three to four dTMP-PCP are bound before chase was added (•). Two dTMP-PCPs exchanged with unlabeled dTTP in the medium (○) with a rate constant of 0.002 ± 0.0004 s−1; one dTMP-PCP did not exchange even after 50-min chase time. (Inset) dTTP hydrolysis in the presence of dTMP-PCP. Reaction conditions were same as in C, except 4A′ was preincubated with dTMP-PCP and hydrolysis of 5 mM [α-32P]dTTP was measured. dTTP was hydrolyzed without lag at 0.1 ± 0.001 s−1 in the presence of dTMP-PCP (▴) and at 0.14 ± 0.003 s−1 in the absence of dTMP-PCP (♦).