Abstract
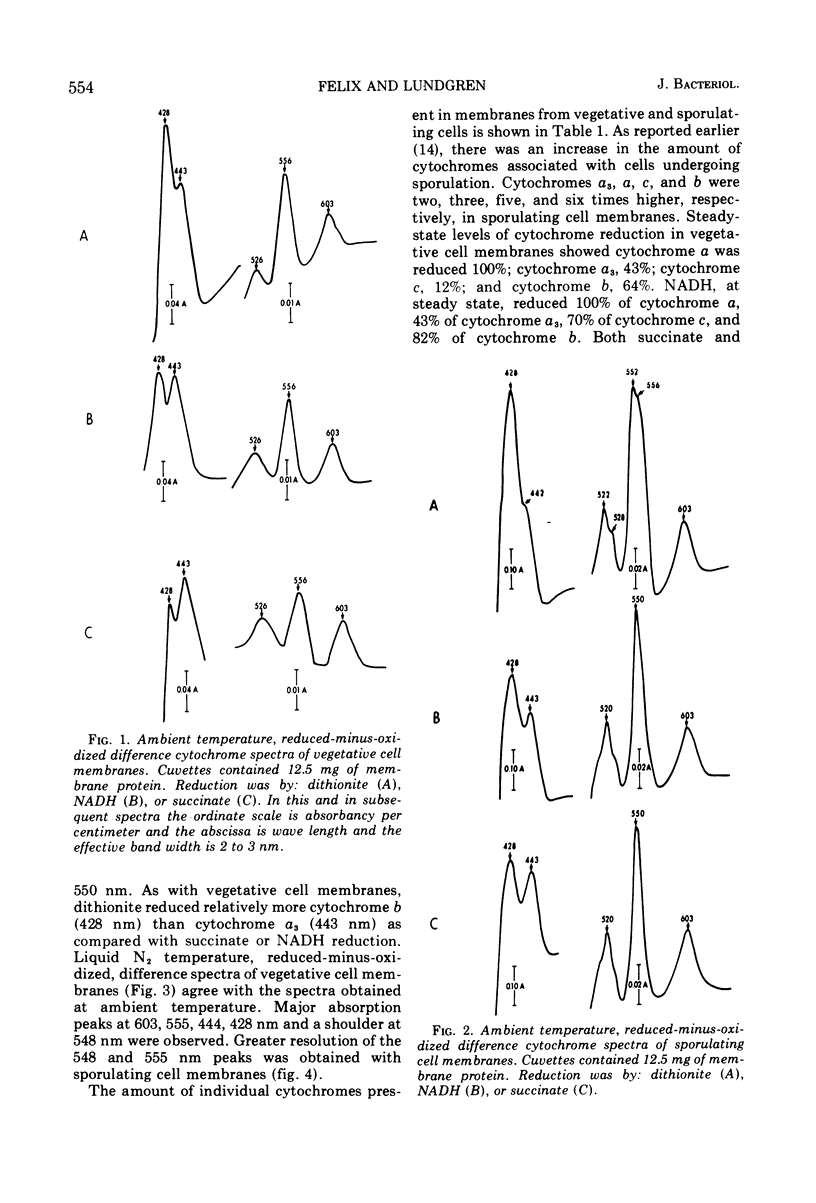
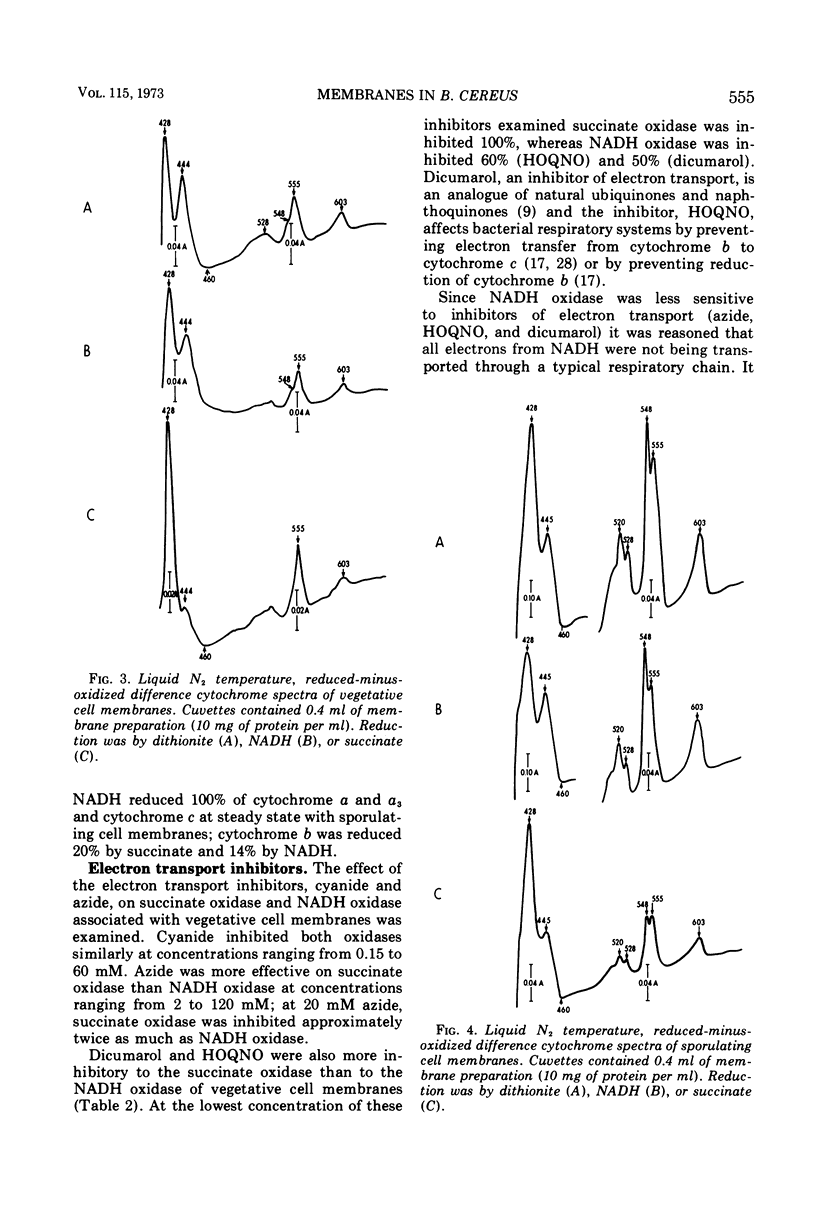
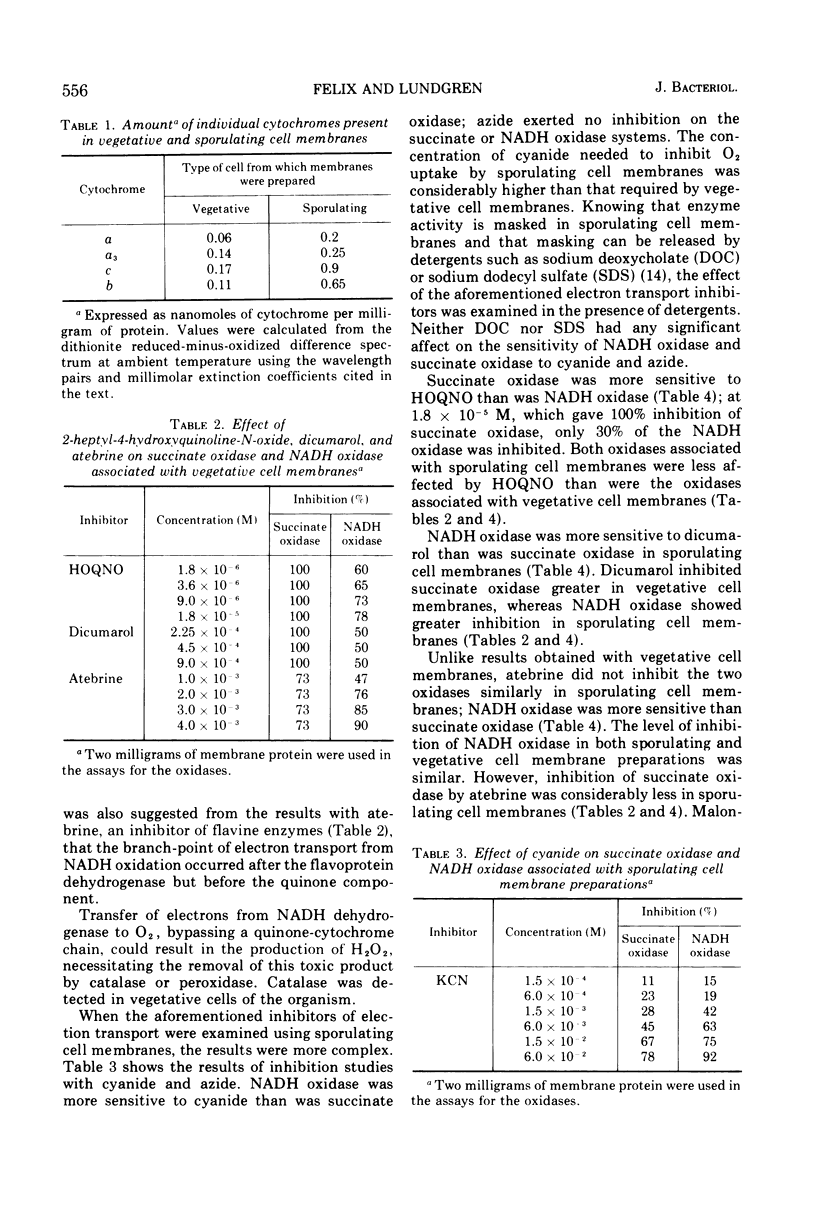
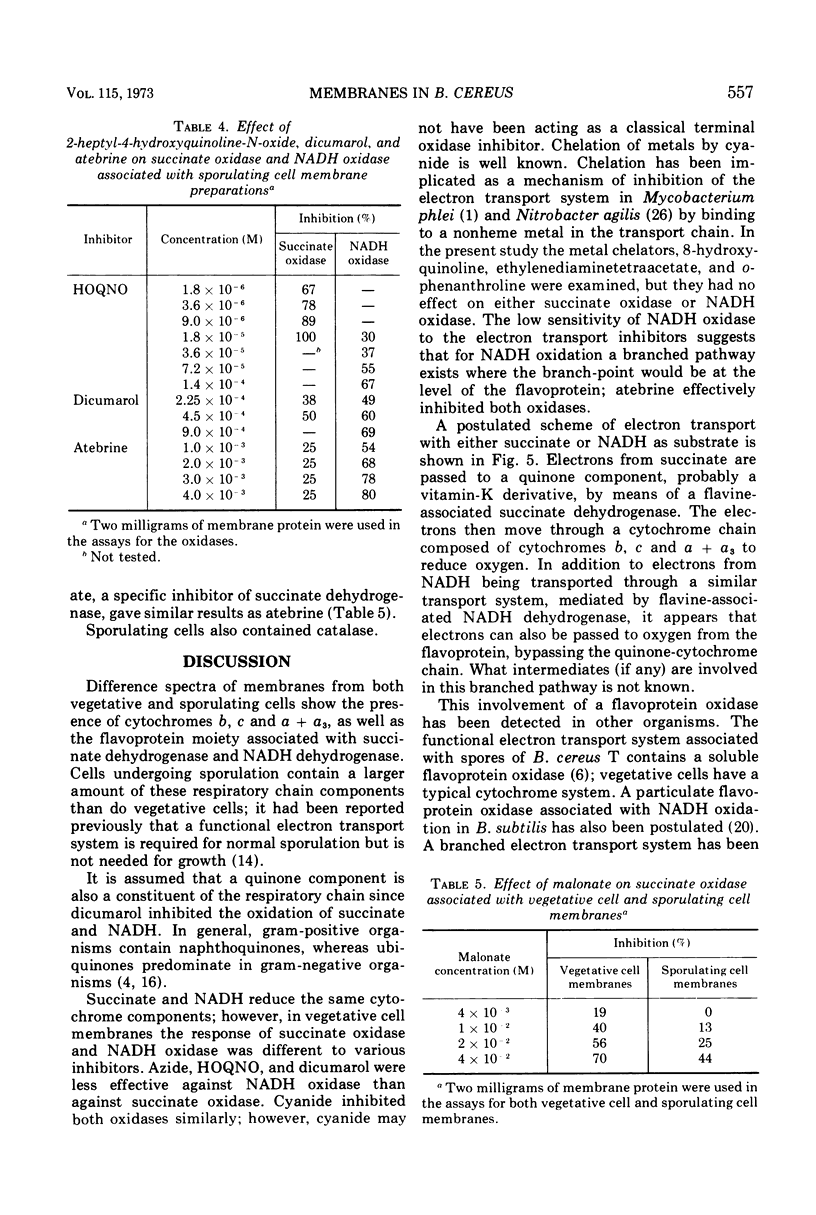
Membranes isolated from Bacillus cereus ATCC 4342 during vegetative growth and during sporulation contained cytochromes b, c and a + a3 as well as flavoprotein as determined from reduced-minus-oxidized difference spectra. Although there appeared to be no qualitative change in the cytochromes, there was a significant increase in the amount of cytochromes associated with membranes isolated from sporulating cells. Succinate and nicotinamide adenine dinucleotide (reduced form) (NADH) reduced the same cytochromes indicating similar pathways of electron transport. The electron transport inhibitors—cyanide, azide, 2-heptyl-4-hydroxyquinoline-N-oxide, dicumarol and atebrine—were examined for their effect on succinate oxidase (succinate: [O2] oxidoreductase) and NADH oxidase (NADH: [O2] oxidoreductase). NADH oxidase associated with vegetative cell membranes was less sensitive to certain inhibitors than was succinate oxidase, suggesting a branched electron transport pathway for NADH oxidation. In addition to electrons being passed to O2 through a quinone-cytochrome chain, it appears that these intermediate carriers can be bypassed such that O2 is reduced by electrons mediated by NADH dehydrogenase. Both oxidases associated with sporulating cell membranes were inhibited to a lesser degree than were the oxidases associated with vegetative cell membranes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASANO A., BRODIE A. F. OXIDATIVE PHOSPHORYLATION IN FRACTIONATED BACTERIAL SYSTEMS. XIV. RESPIRATORY CHAINS OF MYCOBACTERIUM PHLEI. J Biol Chem. 1964 Dec;239:4280–4291. [PubMed] [Google Scholar]
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Electron transport particles released upon lysis of spheroplasts of Escherichia coli B by Brij 58. Biochim Biophys Acta. 1970 Sep 1;216(2):250–261. doi: 10.1016/0005-2728(70)90216-1. [DOI] [PubMed] [Google Scholar]
- Buono F., Testa R., Lundgren D. G. Physiology of growth and sporulation in Bacillus cereus. I. Effect of glutamic and other amino acids. J Bacteriol. 1966 Jun;91(6):2291–2299. doi: 10.1128/jb.91.6.2291-2299.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R. H., HALVORSON H. Comparison of electron transport systems in vegetative cells and spores of Bacillus cereus. J Bacteriol. 1961 Jan;81:51–58. doi: 10.1128/jb.81.1.51-58.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M., Storey B. T. The Respiratory Chain of Plant Mitochondria: VII. Kinetics of Flavoprotein Oxidation in Skunk Cabbage Mitochondria. Plant Physiol. 1970 Oct;46(4):618–624. doi: 10.1104/pp.46.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- LIGHTBOWN J. W., JACKSON F. L. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1956 May;63(1):130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. R., Felix J., Lundgren D. G. Development of a membrane-bound resiratory system prior to and during sporulation in Bacillus cereus and its relationship to membrane structure. J Bacteriol. 1972 Jun;110(3):968–977. doi: 10.1128/jb.110.3.968-977.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. R., Lundgren D. G. Lipid composition of Bacillus cereus during growth and sporulation. J Bacteriol. 1970 Feb;101(2):483–489. doi: 10.1128/jb.101.2.483-489.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Ulrich J. T. Effect of temperature on the respiration and cytochromes of an extreme thermophile. J Bacteriol. 1972 May;110(2):777–779. doi: 10.1128/jb.110.2.777-779.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENKRANZ H. S., BENDICH A. J. STUDIES ON THE BACTERIOSTATIC ACTION OF HYDROXYLAMINE. Biochim Biophys Acta. 1964 May 18;87:40–53. doi: 10.1016/0926-6550(64)90045-3. [DOI] [PubMed] [Google Scholar]
- STRAAT P. A., NASON A. CHARACTERIZATION OF A NITRATE REDUCTASE FROM THE CHEMOAUTOTROPH NITROBACTER AGILIS. J Biol Chem. 1965 Mar;240:1412–1426. [PubMed] [Google Scholar]
- SZULMAJSTER J., SCHAEFFER P. [Augmentation of the DPNH-oxidase activity during sporulation of Bacillus subtilis]. C R Hebd Seances Acad Sci. 1961 Jan 4;252:220–222. [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. IV. Oxidation rates of the respiratory carriers of mung bean mitochondria in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):447–454. doi: 10.1104/pp.45.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABER H. W., MORRISON M. ELECTRON TRANSPORT IN STAPHYLOCOCCI. PROPERTIES OF A PARTICLE PREPARATION FROM EXPONENTIAL PHASE STAPHYLOCOCCUS AUREUS. Arch Biochem Biophys. 1964 May;105:367–379. doi: 10.1016/0003-9861(64)90021-9. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Epel D. The cytochrome system of sea urchin sperm. Arch Biochem Biophys. 1968 Jul;126(1):83–90. doi: 10.1016/0003-9861(68)90562-6. [DOI] [PubMed] [Google Scholar]
- van Gelder B. F. On cytochrome c oxidase. I. The extinction coefficients of cytochrome a and cytochrome a3. Biochim Biophys Acta. 1966 Apr 12;118(1):36–46. doi: 10.1016/s0926-6593(66)80142-x. [DOI] [PubMed] [Google Scholar]


