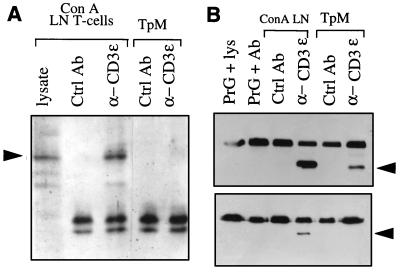
Figure 4.
The TCRζ-chain and CD3ɛ are not tyrosine-phosphorylated in TpM T cells. (A) The TCRζ-chain is not tyrosine-phosphorylated in TpM T cells. The TCR/CD3 complex was immunoprecipitated from Brij96 lysates prepared from TpM or ConA-stimulated LN T cells, using an anti-CD3ɛ mAb. Control immunoprecipitations were carried out using a mAb directed against CD8 (Ctrl Ab). Immunoprecipitated proteins were separated by electrophoresis under nonreducing conditions and analyzed by Western blot using an antiphosphotyrosine mAb (PY20). Whole cell lysate from ConA-stimulated LN T cells was loaded as a control. The position of the 34-kDa TCRζ-chain dimer is indicated by an arrow. The doublets are protein G. (B) The CD3ɛ chain of TpM T cells is not tyrosine-phosphorylated. CD3ɛ was immunoprecipitated directly from TrX-100 lysates of TpM or ConA-stimulated LN T cells using either an anti-CD3ɛ mAb (α-CD3) or a control mAb directed against bovine γδ TCR (Ctrl Ab). Further controls included protein G-Sepharose incubated with anti-CD3ɛ mAb alone (PrG + Ab) or protein G-Sepharose incubated with lysate of ConA-stimulated LN T cells in the absence of antibody (PrG + lys). Immunoprecipitated proteins were divided into two equal fractions and analysis by Western blot using anti-human CD3ɛ, recognizing the cytoplasmic region of the bovine CD3ɛ (Upper), or anti-phosphotyrosine antibody PY20 (α-PY, Lower). The position of the 24-kDa CD3ɛ is indicated by arrows. The top bands are protein G.