Abstract
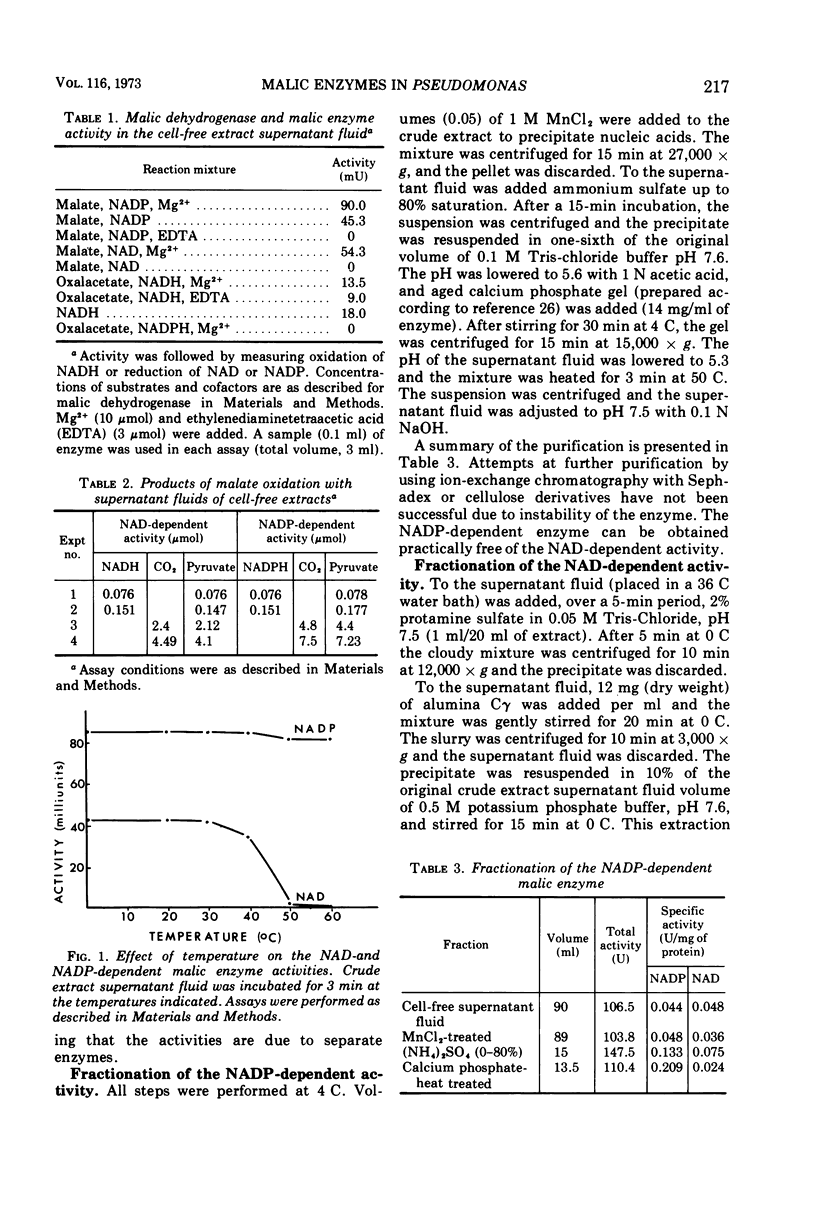
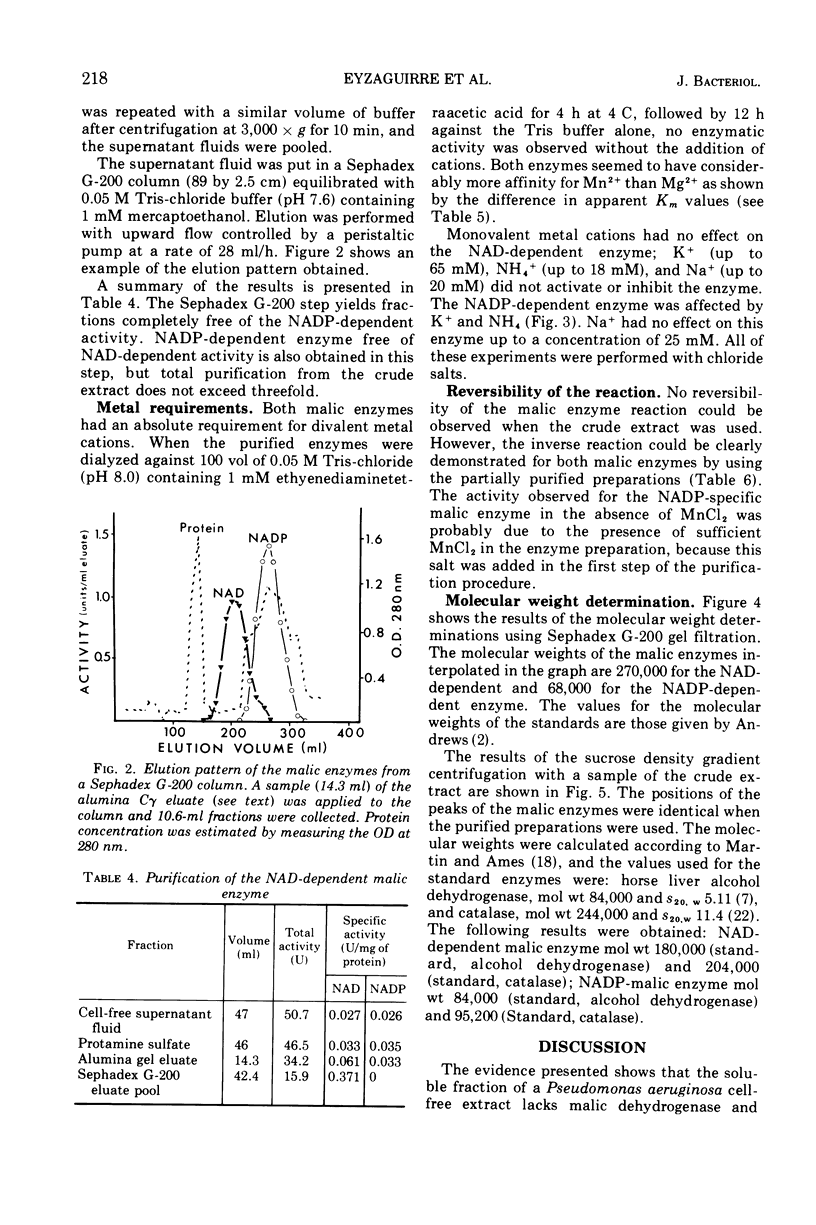
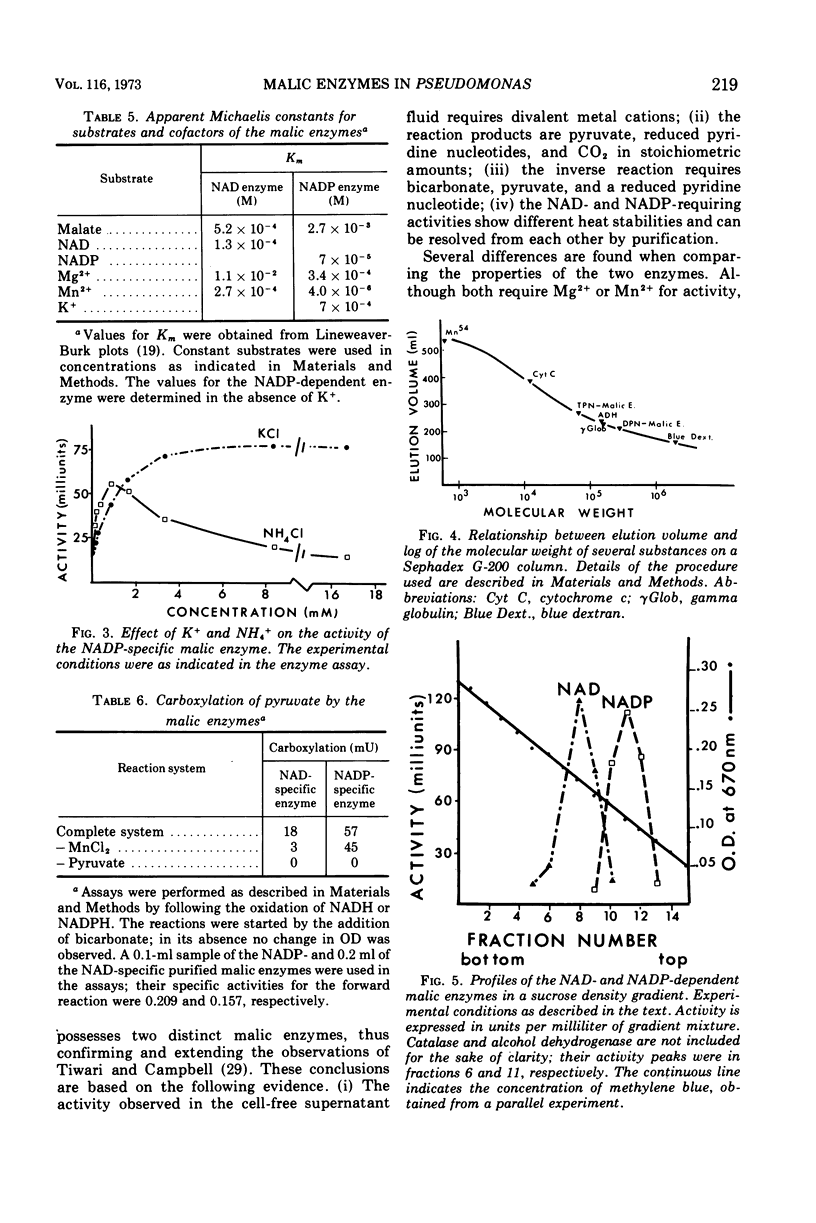
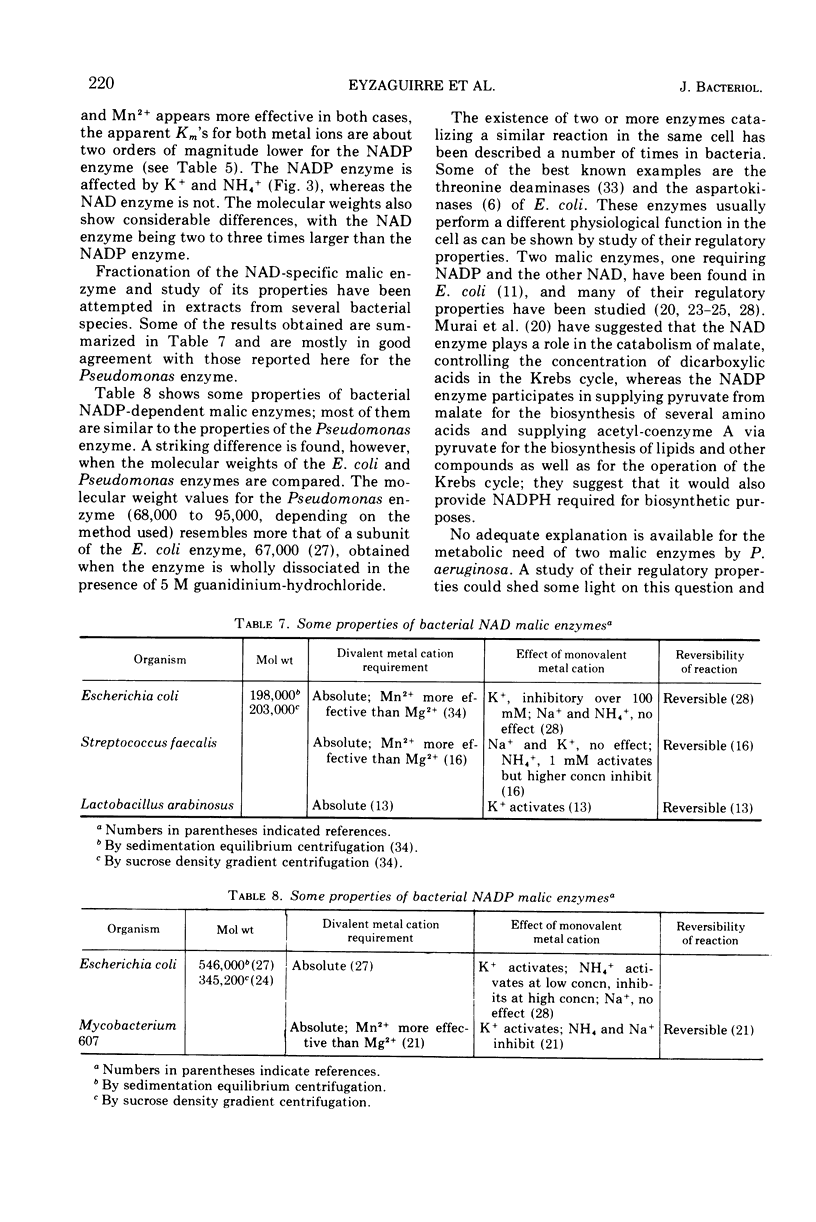
Cell-free extract supernatant fluids of Pseudomonas aeruginosa were shown to lack malic dehydrogenase but possess a nicotinamide adenine dinucleotide (NAD)- or NAD phosphate (NADP)-dependent enzymatic activity, with properties suggesting a malic enzyme (malate + NAD (NADP) → pyruvate + reduced NAD (NADH) (reduced NADP [NADPH] + CO2), in agreement with earlier findings. This was confirmed by determining the nature and stoichiometry of the reaction products. Differences in heat stability and partial purification of these activities demonstrated the existence of two malic enzymes, one specific for NAD and the other for NADP. Both enzymes require bivalent metal cations for activity, Mn2+ being more effective than Mg2+. The NADP-dependent enzyme is activated by K+ and low concentrations of NH4+. Both reactions are reversible, as shown by incubation with pyruvate, CO2, NADH, or NADPH and Mn2+. The molecular weights of the enzymes were estimated by gel filtration (270,000 for the NAD enzyme and 68,000 for the NADP enzyme) and by sucrose density gradient centrifugation (about 200,000 and 90,000, respectively).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Cazzulo J. J., Massarini E. Inhibition of NADP-linked malic enzyme by glyoxylate. FEBS Lett. 1972 Apr 15;22(1):76–79. doi: 10.1016/0014-5793(72)80223-0. [DOI] [PubMed] [Google Scholar]
- Fields G. A., Bright H. J. Magnetic resonance and kinetic studie of the activation of beta-methylaspartase by manganese. Biochemistry. 1970 Sep 15;9(19):3801–3809. doi: 10.1021/bi00821a020. [DOI] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J., Dagley S. Metabolism of l-Malate and d-Malate by a Species of Pseudomonas. J Bacteriol. 1970 Dec;104(3):1197–1202. doi: 10.1128/jb.104.3.1197-1202.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L. A., Bartholomaus R. C., Gunsalus I. C. Repression of malic enzyme by acetate in Pseudomonas. Biochem Biophys Res Commun. 1966 Sep 22;24(6):955–960. doi: 10.1016/0006-291x(66)90343-3. [DOI] [PubMed] [Google Scholar]
- KAUFMAN S., KORKES S., DEL CAMPILLO A. Biosynthesis of dicarboxylic acids by carbon dioxide fixation. V. Further study of the "malic" enzyme of Lactobacillus arabinosus. J Biol Chem. 1951 Sep;192(1):301–312. [PubMed] [Google Scholar]
- KORKES S., DEL CAMPILLO A., OCHOA S. Biosynthesis of dicarboxylic acids by carbon dioxide fixation. IV. Isolation and properties of an adaptive "malic" enzyme from Lactobacillus arabinosus. J Biol Chem. 1950 Dec;187(2):891–905. [PubMed] [Google Scholar]
- KORNBERG H. L., MADSEN N. B. The metabolism of C2 compounds in microorganisms. 3. Synthesis of malate from acetate via the glyoxylate cycle. Biochem J. 1958 Mar;68(3):549–557. doi: 10.1042/bj0680549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H., Takeo K., Kameda K., Tanaka S. Existence of two malic enzymes in Escherichia coli. Biochem Biophys Res Commun. 1967 May 5;27(3):331–336. doi: 10.1016/s0006-291x(67)80102-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus: physiological properties and purification of an inducible malic enzyme. J Bacteriol. 1969 May;98(2):705–711. doi: 10.1128/jb.98.2.705-711.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Matula T. I., McDonald I. J., Martin S. M. CO2 fixation by malic enzyme in a species of Micrococcus. Biochem Biophys Res Commun. 1969 Mar 31;34(6):795–802. doi: 10.1016/0006-291x(69)90250-2. [DOI] [PubMed] [Google Scholar]
- Murai T., Tokushige M., Nagai J., Katsuki H. Studies on regulatory functions of malic enzymes. I. Metabolic functions of NAD- and NADP-linked malic enzymes in Escherichia coli. J Biochem. 1972 Jun;71(6):1015–1028. doi: 10.1093/oxfordjournals.jbchem.a129850. [DOI] [PubMed] [Google Scholar]
- PARVIN R., PANDE S. V., VENKITASUBRAMANIAN T. A. PURIFICATION AND PROPERTIES OF MALATE DEHYDROGENASE (DECARBOXYLATING) FROM MYCOBACTERIUM 607. Biochim Biophys Acta. 1964 Nov 22;92:260–277. doi: 10.1016/0926-6569(64)90184-1. [DOI] [PubMed] [Google Scholar]
- SAMEJIMA T., YANG J. T. RECONSTITUTION OF ACID-DENATURED CATALASE. J Biol Chem. 1963 Oct;238:3256–3261. [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. The L-amino acid oxidases of snake venom. II. Isolation and characterization of homogeneous L-amino acid oxidase. Arch Biochem. 1950 Nov;29(1):190–209. [PubMed] [Google Scholar]
- Sanwal B. D. Regulatory characteristics of the diphosphopyridine nucleotide-specific malic enzyme of Escherichia coli. J Biol Chem. 1970 Mar 10;245(5):1212–1216. [PubMed] [Google Scholar]
- Sanwal B. D., Smando R. Malic enzyme of Escherichia coli. Diversity of the effectors controlling enzyme activity. J Biol Chem. 1969 Apr 10;244(7):1817–1823. [PubMed] [Google Scholar]
- Sanwal B. D., Smando R. Malic enzyme of Escherichia coli. Possible mechanism for allosteric effects. J Biol Chem. 1969 Apr 10;244(7):1824–1830. [PubMed] [Google Scholar]
- Takeo K. Existence and properties of two malic enzymes in Escherichia coli especially of NAD-linked enzyme. J Biochem. 1969 Sep;66(3):379–387. doi: 10.1093/oxfordjournals.jbchem.a129156. [DOI] [PubMed] [Google Scholar]
- Tiwari N. P., Campbell J. J. Utilization of dicarboxylic acids by Pseudomonas aeruginosa. Can J Microbiol. 1969 Sep;15(9):1095–1100. doi: 10.1139/m69-194. [DOI] [PubMed] [Google Scholar]
- Vidal M. C., Cazzulo J. J. Allosteric inhibition of NADP-linked malic enzyme from an extreme halophile by acetyl-CoA. FEBS Lett. 1972 Oct 1;26(1):257–260. doi: 10.1016/0014-5793(72)80586-6. [DOI] [PubMed] [Google Scholar]
- Von Tigerstrom M., Campbell J. J. The tricarboxylic acid cycle, the glyoxylate cycle, and the enzymes of glucose oxidation in Pseudomonas aeruginosa. Can J Microbiol. 1966 Oct;12(5):1015–1022. doi: 10.1139/m66-136. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Tokushige M., Katsuki H. Studies on regulatory functions of malic enzymes. II. Purification and molecular properties of nicotinamide adenine dinucleotide-linked malic enzyme from Eschericha coli. J Biochem. 1973 Jan;73(1):169–180. [PubMed] [Google Scholar]


