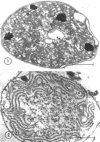
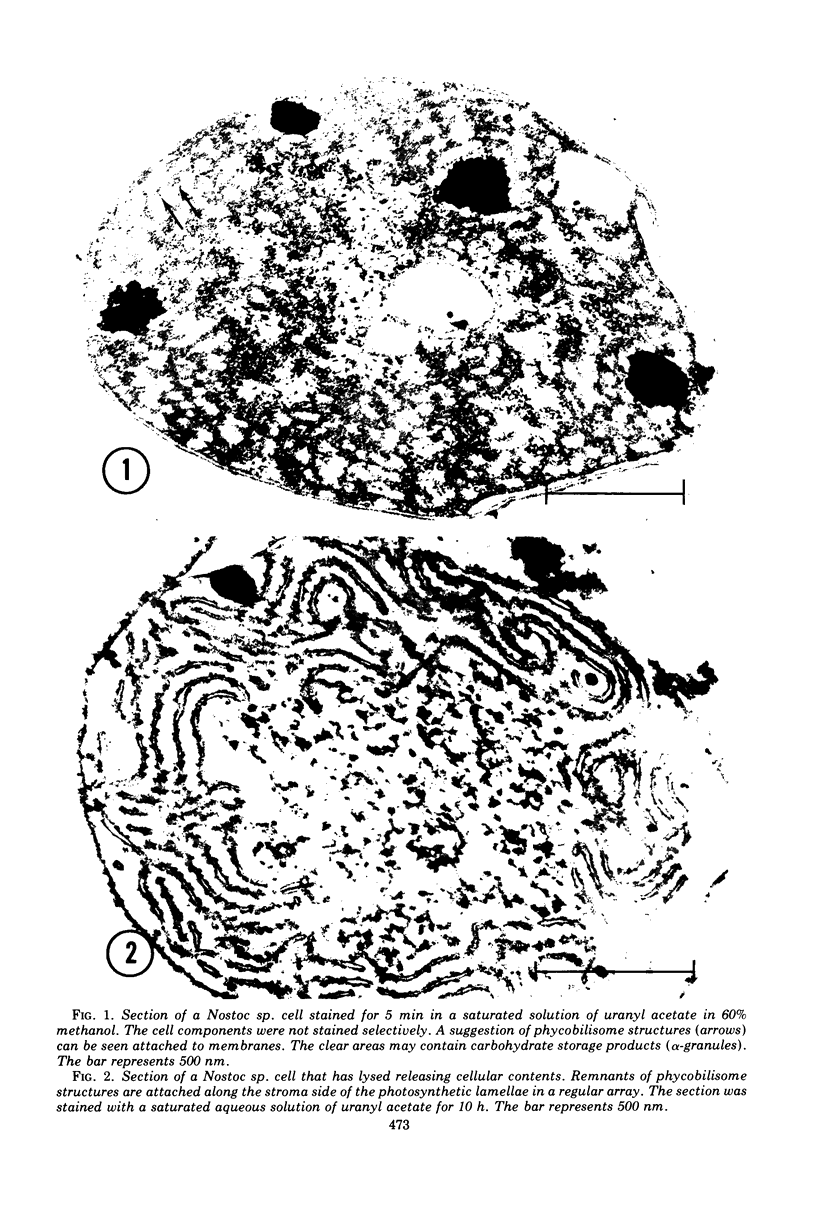
Abstract
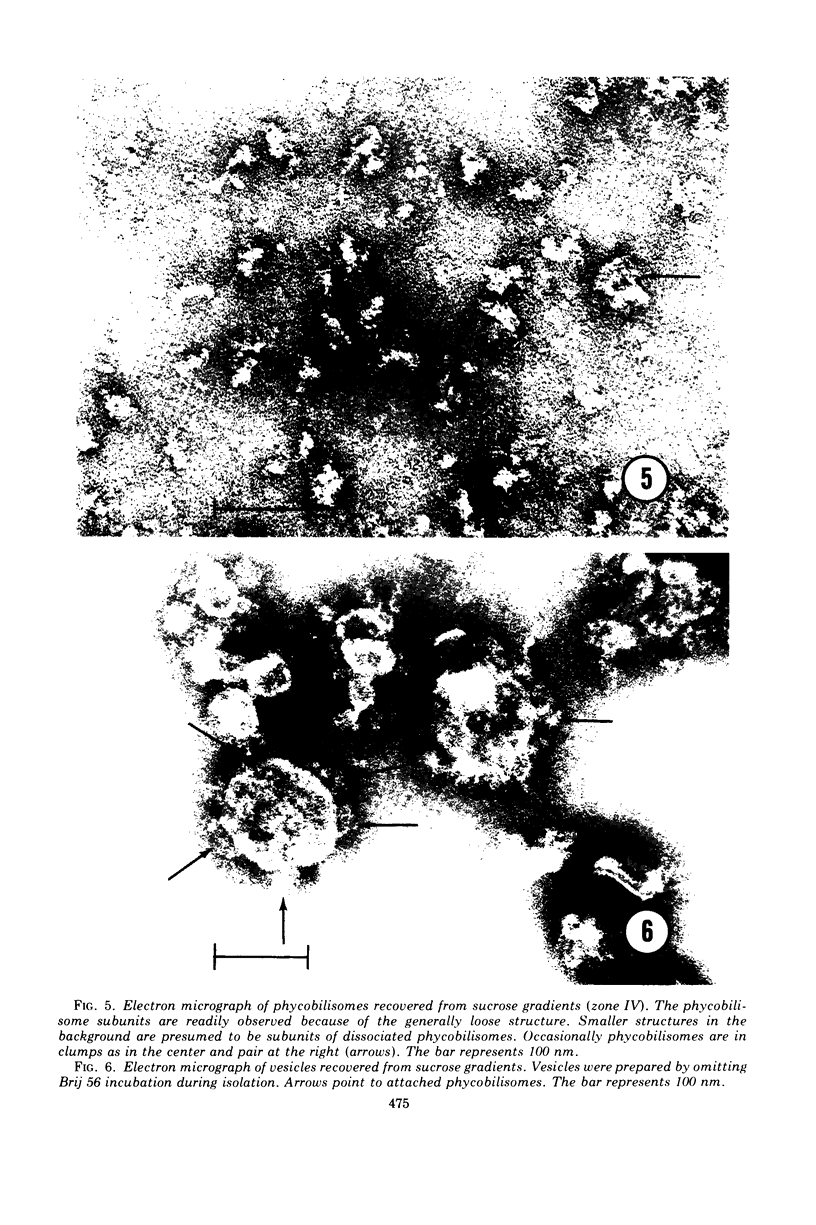
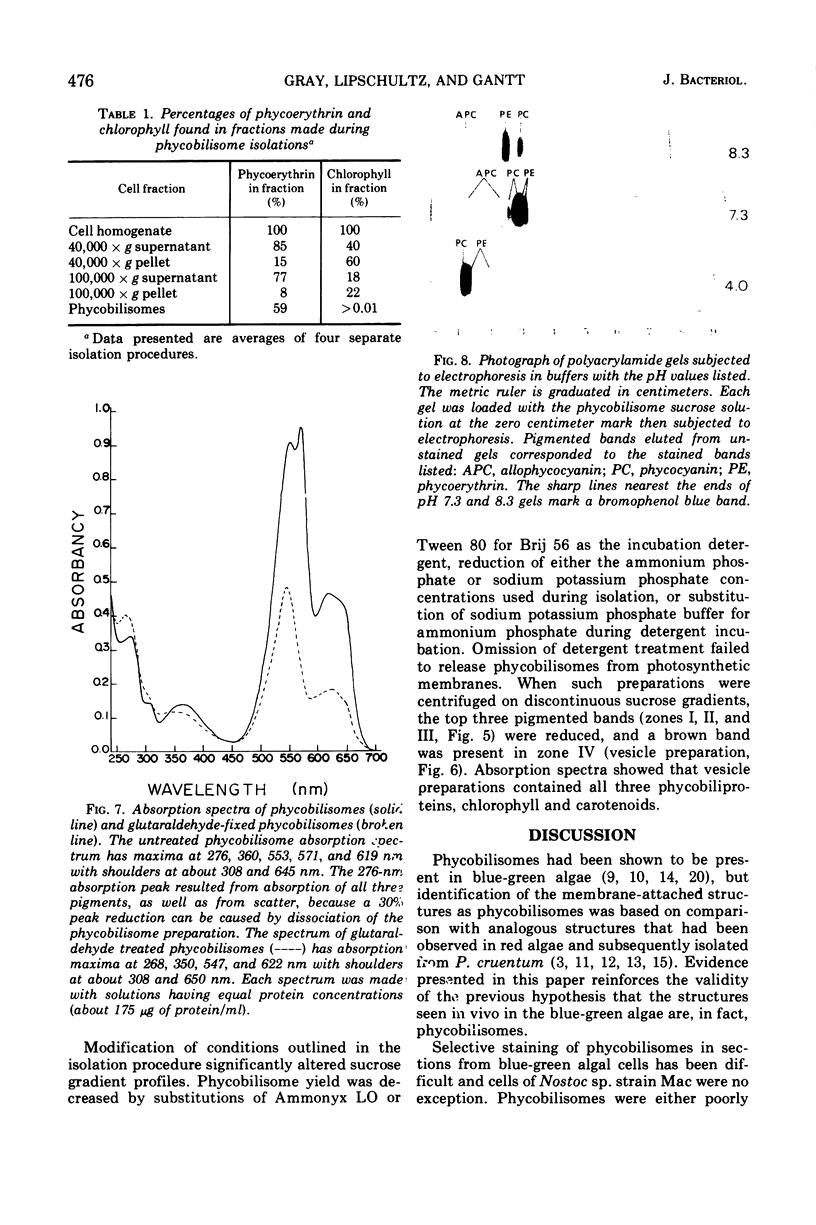
Phycobilisomes were isolated from a Nostoc sp. strain Mac in phosphate buffer (pH 7.0) by treatment with 1% Brij 56 and centrifugation on discontinuous sucrose gradients (2.0, 1.0, 0.5, and 0.25 M in the proportions 6:4:4:10 ml, respectively). Absorption spectra of isolated phycobilisomes showed the presence of phycoerythrin, phycocyanin, and allophycocyanin. The phycobilisome pigments were partially resolved by electrophoresis on acrylamide gels. Stained gels demonstrated that each main protein band corresponded to a pigmented region. The phycobilisomes appeared compact with a rounded surface and flattened base (about 40-nm diameter) at the attachment site to the photosynthetic lamellae. Fixation in glutaraldehyde caused a significant reduction in total pigment absorption, as well as shifts in the absorption maxima, particularly that of phycoerythrin.
Full text
PDF

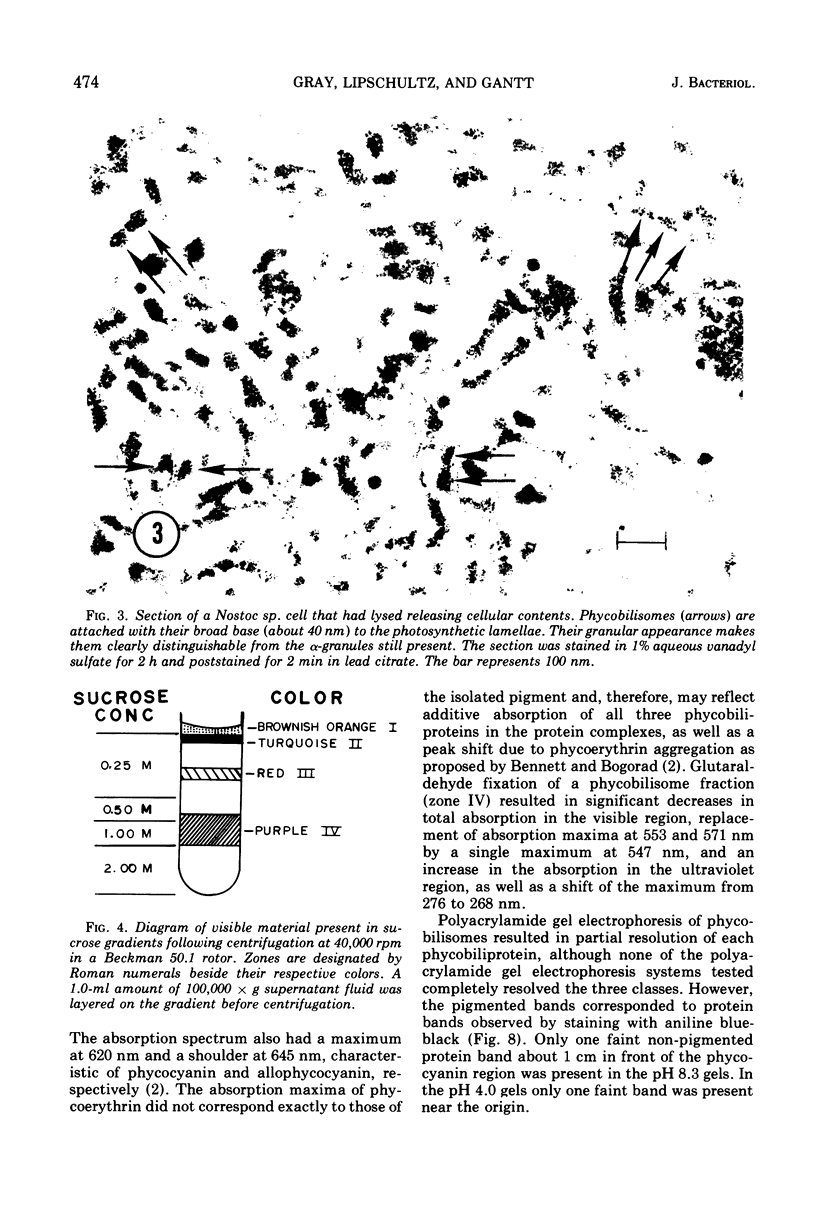


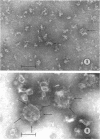
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNOLD W., OPPENHEIMER J. R. Internal conversion in the photosynthetic mechanism of blue-green algae. J Gen Physiol. 1950 Mar;33(4):423–435. doi: 10.1085/jgp.33.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Properties of subunits and aggregates of blue-green algal biliproteins. Biochemistry. 1971 Sep 14;10(19):3625–3634. doi: 10.1021/bi00795a022. [DOI] [PubMed] [Google Scholar]
- Bisalputra T., Bisalputra A. A. The occurrence of DNA fibrils in chloroplasts of Laurencia spectabilis. J Ultrastruct Res. 1967 Jan;17(1):14–22. doi: 10.1016/s0022-5320(67)80016-9. [DOI] [PubMed] [Google Scholar]
- Bowyer J. W., Skerman V. B. Production of axemic cultures of soil-borne and endophytic blue-green algae. J Gen Microbiol. 1968 Dec;54(2):299–306. doi: 10.1099/00221287-54-2-299. [DOI] [PubMed] [Google Scholar]
- Clement-Metral J. D., Lefort-Tran M. Fluorescence transfer in glutaraldehyde fixed particles of the red alga Porphyridium cruentum (N). FEBS Lett. 1971 Jan 25;12(4):225–228. doi: 10.1016/0014-5793(71)80026-1. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Lefort-Tran M. Fixation of phycobiliproteins to photosynthetic membranes by glutaraldehyde. Arch Mikrobiol. 1970;71(3):245–257. doi: 10.1007/BF00410158. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N. M. Transfer of light energy within the pigment systems present in photosynthesizing cells. Nature. 1951 Sep 29;168(4274):548–550. doi: 10.1038/168548a0. [DOI] [PubMed] [Google Scholar]
- Evans E. L., Allen M. M. Phycobilisomes in Anacystis nidulans. J Bacteriol. 1973 Jan;113(1):403–408. doi: 10.1128/jb.113.1.403-408.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Granules associated with the chloroplast lamellae of Porphyridium cruentum. J Cell Biol. 1966 Jun;29(3):423–434. doi: 10.1083/jcb.29.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Phycobiliprotein localization in algae. Brookhaven Symp Biol. 1966;19:393–405. [PubMed] [Google Scholar]
- Gantt E., Conti S. F. The ultrastructure of Porphyridium cruentum. J Cell Biol. 1965 Aug;26(2):365–381. doi: 10.1083/jcb.26.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Ultrastructure of blue-green algae. J Bacteriol. 1969 Mar;97(3):1486–1493. doi: 10.1128/jb.97.3.1486-1493.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Energy transfer in phycobilisomes from phycoerythrin to allophycocyanin. Biochim Biophys Acta. 1973 Apr 5;292(3):858–861. doi: 10.1016/0005-2728(73)90036-4. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Phycobilisomes of Porphyridium cruentum. I. Isolation. J Cell Biol. 1972 Aug;54(2):313–324. doi: 10.1083/jcb.54.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Van Baalen C. Characteristics of a stable, filamentous mutant of a coccoid blue-green alga. J Bacteriol. 1970 Jun;102(3):784–789. doi: 10.1128/jb.102.3.784-789.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]