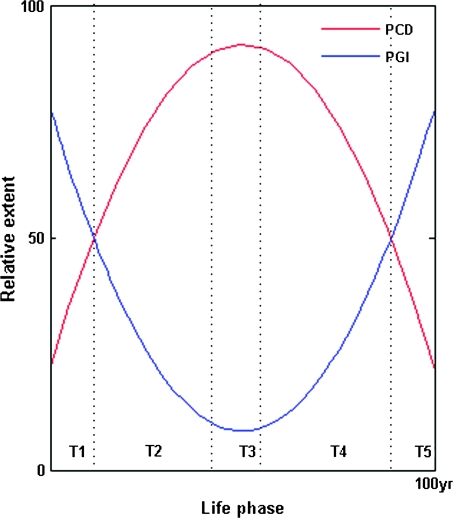
FIG. 6.—
Model of changing human male CT and GK function from germ line to somatic contexts. The x axis is divided into the following time frames: T1, prezygotic spermatogonia and spermatocytes; T2, postzygotic embryonic development; T3, prereproductive infancy and childhood; T4, reproductive adult life; and T5, postreproductive senescence. The y axis models the relative extent of either GK functionality (PCD) or CT dysfunctionality (PGI) during these time frames. Damage/stress-inducible prezygotic promoter (CpG island) methylation of spermatogonial/spermatocyte CTs and GKs, respectively, increases PGI while reducing PCD—thus maximizing genetic variability in response to changing environmental selection pressures. Postfertilization male germ line gene demethylation has the opposite effect, causing a sustained decline in PGI and a rise in PCD. During postreproductive adult life, an age-dependent (as well as damage-inducible) methylation clock induces somatic CT/GK gene inactivation, leading in turn to a senescent rise in PGI and decline of PCD that predisposes to sporadic tumor outgrowth.