Abstract
Objective
To determine the effects of ibuprofen on serum electrolyte concentrations after a 160 km running race.
Methods
Twenty nine subjects (mean (SD) age 47.9 (7.4) years) ingested 600 mg ibuprofen the day before, and 1200 mg ibuprofen during, a 160 km competitive trail running race (approximately every 4 h in 200 mg doses). Twenty five control subjects (mean (SD) age 46.8 (10.3) years) avoided ingestion of ibuprofen before or during the race. Blood was drawn on the day before the race and immediately after the race. Serum biochemical profiles were analysed by a clinical laboratory. Significant effects of treatment and time were determined with a general linear model with repeated measures.
Results
Subjects in the two groups did not differ by age, training volume, race experience, body mass index, body fat, or finishing time (25.8 (3.3) vs 25.6 (3.9) h). Body weight did not change significantly over the race (measured before, mid‐race (90 km), and after). Ibuprofen ingestion did not significantly affect any of the serum markers including creatine kinase (p = 0.16). A significant decrease in serum sodium (p = 0.006), potassium (p = 0.001), chloride (p<0.001), calcium (p<0.001), albumin (p<0.001) and globulin (p<0.001) was observed after the race. Increases were seen in creatine kinase (p<0.001), creatinine (p<0.001), blood urea nitrogen (p<0.001), uric acid (p<0.001) and glucose (p<0.001) as the result of the race.
Conclusions
These data suggest that the non‐specific cyclo‐oxygenase inhibitor, ibuprofen, does not alter serum electrolyte concentrations during ultradistance running. However, the stress of ultradistance running appears to be related to significant changes in certain serum markers.
Keywords: ultraendurance running, prolonged exercise, hyponatraemia, non‐steroidal anti‐inflammatory drugs, cyclo‐oxygenase inhibitors
Several reports suggest that ultradistance athletes are at risk of low serum sodium and potentially hyponatraemia,1,2,3,4,5 which may be the result of over‐hydration,6,7,8,9,10,11,12 too little sodium intake,13 or syndrome of inappropriate antidiuresis.14,15,16 An additional factor may be use of non‐steroidal anti‐inflammatory drugs (NSAIDs). It has been reported that as many as 75% of ultradistance athletes use NSAIDs during competition.6,17,18,19 Indeed, NSAIDs have been implicated in the causation of hyponatraemia or low serum sodium independent of exercise.20,21,22,23,24,25 Three studies have investigated the effects of retrospective pre‐exercise NSAID use on serum biochemical compounds after a marathon.6,26,27 No studies to date have demonstrated the effects of NSAIDs in a dose‐controlled intervention experimental design during an ultradistance running race.
NSAIDs have long been suspected of adversely affecting renal function.28,29 However, this is not a consistent finding and may be only in the renally compensated.22,29,30,31 NSAIDs may act as antidiuretics during exercise, possibly through the inhibition of prostaglandin formation which typically inhibits arginine vasopressin (AVP).25,32 This interaction between NSAIDs and the antidiuretic hormone, AVP, which is raised during exercise, may thus contribute to water retention.6,25,31 There is also evidence that NSAIDs may inhibit diuresis independent of antidiuretic hormone through the inhibition of renal prostaglandins.33 Water retention, typically assessed by weight gain in field research, has been tightly linked with decreases in serum sodium and outright hyponatraemia.8,10,11,12,34,35,36,37 It has been reported that ad libitum intake of NSAIDs probably results in altered serum electrolytes in a marathon cohort6,26; however, findings are not consistent.27 Wharam et al19 showed in a retrospective study that ironman triathletes who chose to use NSAIDs (30%) in the 24 h before a race had increased plasma potassium and creatinine and reduced sodium after the race. It has also been shown that NSAID use increases seven cytokines after ultradistance running,17 and, more recently, an increase in liver, but not muscle, damage markers has been found.38 Thus it is the purpose of this research to illustrate the effect of a prescribed dose of ibuprofen on serum electrolytes after a 160 km running race with an intervention experimental design.
Methods
Subject selection
Sixty three experienced male and female ultramarathoners from the 2005 Western States 100 Mile Endurance Run were recruited and provided pre‐race blood samples. Athletes were placed into an ibuprofen (n = 33) or a control (n = 30) group on the basis of their willingness to use or avoid ibuprofen before and during the race. Permission for a randomised, placebo‐controlled research design was not granted by the race medical board because of concerns about compliance by athletes suffering from pain during the later stages of the 20–30 h race. Fifty four subjects (29 in the ibuprofen group, 25 in the control group) completed the race and provided post‐race blood samples. Informed consent was obtained from each subject, and the experimental procedures were in accordance with the policy statements of the institutional review board of Appalachian State University and the Declaration of Helsinki. To enter the study, runners must have qualified for the 2005 160 km Western States Endurance Run.
Race description
Qualification for the Western States Endurance Run requires that runners must have completed a 160 km race, a 100 km race in less than 14 h, or a 50 mile run in less than 11 h and be chosen in a lottery 6 months before the race.
The 160 km Western States Endurance Run is a point‐to‐point trail run in the Sierra Nevada Mountains of northern California, and is regarded as one of the most difficult ultradistance trail races in the United States. The race starts at Squaw Valley, California (1890 m altitude), and finishes at Auburn, California (366 m). The course ascends 777 m to Emigrant Pass (2668 m, the highest point) within the first 7 km and then passes through remote and rugged territory. The total altitude gain and loss during the race has been estimated at 5500 m and 6700 m, respectively. The race starts at 05:00, and runners must reach the finish line within 30 h to be considered an official finisher.
Research design
Subjects provided blood during registration on the morning before the race. Pre‐race body mass and percentage body fat (three‐site skinfolds) was measured, and subjects filled in a questionnaire on basic demographics and training history during training. Subjects in the ibuprofen group ingested 600 mg (three 200 mg tablets) during the afternoon before the race, and 1200 mg on race day (six 200 mg tablets, with one taken before the race and one approximately every 4 h thereafter). Subjects in the control group avoided use of all drugs including ibuprofen, and subjects in the ibuprofen group avoided all other drug use. On race day, body mass was measured at the 90 km aid station (Michigan Bluff, 1220 m) and within 5–10 min of the finish of the race. Subjects completed a post‐race questionnaire indicating adherence to the research design. Subjects from both the control and ibuprofen groups reported complete compliance. Subjects consumed food and beverages ad libitum during the race.
Blood cell counts, diagnostic biochemistry profile and creatine kinase
Blood samples were drawn from the antecubital vein with subjects in the seated position. Complete blood counts and differentials were measured using a Coulter STKS instrument (Coulter Electronics, Inc, Hialeah, Florida, USA). The comprehensive diagnostic biochemistry panel and creatine kinase were measured in a clinical laboratory using an LX‐20 clinical analyser (Beckman, Brea, California, USA). Plasma volume changes were estimated using the method of Dill and Costill.39 Additional data from this study have been published separately.38
Statistical analysis
Data are expressed as mean (SD). Serum markers in the two groups were compared using a general linear model with repeated measures. Comparisons between genders were conducted using Student's t tests. Gender effects on pre‐race and post‐race serum markers were examined, when significant; the general linear model repeated measures were rerun with gender as a covariate. Pearson product–moment correlations were used to test the relationship between changes in measured outcomes. Significance was set at p⩽0.05.
Results
Fifty four of 63 subjects completed the 160 km race (86%). Air temperature was 9°C and humidity was 70% at the start of the race (Squaw Valley), 30°C by 13:00 (Michigan Bluff) with 34% humidity, 14°C and 76% humidity by 02:00, and 25.5°C with 50% humidity by the race cutoff time of 12:00 (Auburn). Table 1 compares the subject characteristics for the ibuprofen (n = 29) and control (n = 25) groups; there were no significant differences in age, body composition, training and racing history, or race time. Male (n = 43) and female (n = 11) runners did not differ significantly in any of the subject characteristics in table 1 except for those related to weight and body composition. There was no significant treatment or time effect on body weight during the race. Plasma volume did not change appreciably, and did not differ significantly between groups (–1.6 (0.4)% and –1.2 (0.3)%, respectively; p = 0.381).
Table 1 Subject characteristics in the ibuprofen (n = 29) and control (n = 25) groups.
| Variable | Ibuprofen | Control |
|---|---|---|
| Age (years) | 47.9 (7.4) | 46.8 (10.3) |
| Height (m) | 1.75 (0.08) | 1.77 (0.09) |
| Pre‐race weight (kg) | 71.0 (11.1) | 71.2 (9.8) |
| 90 km weight (kg) | 71.2 (11.0) | 71.0 (9.3) |
| 160 km weight (kg) | 71.2 (10.9) | 71.1 (9.0) |
| Body fat (%) | 17.8 (6.2) | 16.1 (5.4) |
| Body mass index | 22.8 (2.6) | 22.4 (2.0) |
| Race time (h) | 25.8 (3.3) | 25.6 (3.9) |
| Years running | 16.0 (8.8) | 16.5 (7.3) |
| Ultramarathons raced | 50.6 (67.1) | 41.3 (27.9) |
| Run training (km/week) | 87.1 (6.7) | 76.3 (6.2) |
Values are mean (SD).
Table 2 presents serum markers. There was no significant treatment by time interaction for any of them. There was a significant change over the race in several including increases in creatinine (p<0.001), uric acid (p<0.001), glucose (p = 0.001), blood urea nitrogen (p<0.001), creatine kinase (p<0.001) and bilirubin (p<0.001). Significant decreases after the race were seen in sodium (p = 0.006), potassium (p = 0.001), chloride (p<0.001), calcium (p<0.001), albumin (p<0.001), globulin (p<0.001) and total protein (p<0.001). Significant gender effects on sodium, creatinine and uric acid were found. When analysed with gender as a covariate, significant effects were still seen in creatinine (p<0.001) and uric acid (p = 0.049). However, there was no longer a significant effect on sodium with gender as a covariate (p = 0.83). Nonetheless, a significant treatment by time interaction was not apparent when covaried for gender in any of these serum markers.
Table 2 Serum biochemical profiles in the ibuprofen and control groups.
| Serum marker | Ibuprofen (n = 29, 8 women) | Control (n = 25, 3 women) | Normal range | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Sodium (mmol/l) | 138.8 (1.5) | 137.6 (2.7)* | 139.2 (1.5) | 138.1 (3.1)* | 135–148 |
| Potassium (mmol/l) | 4.13 (0.34) | 3.88 (0.33)*** | 4.14 (0.34) | 3.98 (0.34*** | 3.5–5.5 |
| Chloride (mmol/l) | 104.0 (1.9) | 101.7 (3.0)*** | 104.8 (1.8) | 102.2 (3.8)*** | 96–109 |
| Calcium (mg/dl) | 9.66 (0.25) | 9.37 (0.39)*** | 9.68 (0.32) | 9.40 (0.51)*** | 8.5–10.6 |
| Creatinine (mg/dl) | 0.96 (0.14) | 1.34 (0.28)*** | 1.03 (0.17) | 1.36 (0.30)*** | 0.5–1.5 |
| Glucose (mg/dl) | 101.1 (19.5) | 113.0 (27.1)*** | 89.9 (13.6) | 105.9 (18.9)*** | 65–109 |
| Bilirubin (mg/dl) | 0.90 (0.33) | 1.48 (0.88)*** | 1.03 (0.60) | 1.43 (0.99)*** | 0.1–1.2 |
| ALK (U/l) | 65.7 (14.0) | 64.8 (14.7) | 65.7 (19.6) | 63.8 (15.4) | 25–160 |
| Uric acid (mg/dl) | 4.40 (1.30) | 4.75 (1.40)*** | 4.70 (1.64) | 5.12 (1.77)*** | 2.4–8.2 |
| Total protein (g/dl) | 7.55 (0.49) | 6.92 (0.60)*** | 7.68 (0.59) | 6.97 (0.34)*** | 6.0–8.5 |
| Albumin (g/dl) | 4.65 (0.36) | 4.48 (0.41)*** | 4.78 (0.46) | 4.51 (0.34)*** | 3.5–5.5 |
| Globulin (g/dl) | 2.89 (0.36) | 2.44 (0.34)*** | 2.90 (0.41) | 2.46 (0.31)*** | 1.5–4.5 |
| BUN (mg/dl) | 12.6 (3.6) | 31.4 (12.4) | 13.1 (4.4) | 26.3 (7.9) | 5–25 |
| Creatine kinase (U/l) | 126.5 (57.7) | 20621 (19197) | 156.6 (89.7) | 13886 (15336) | 100–200 |
ALK, alkaline phosphatase; BUN, blood urea nitrogen.
Values are mean (SD).
*p⩽0.05, ***p⩽0.001, compared with before the race.
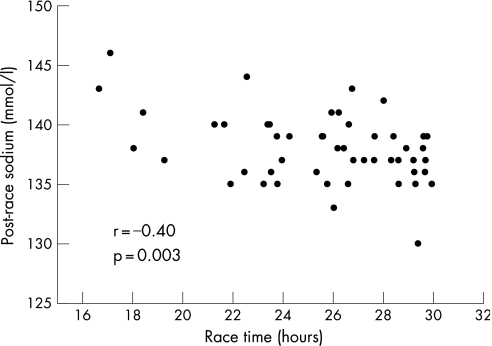
Correlations were observed between the change in sodium over the race with race time (r = 0.37, p = 0.007), post‐race sodium and race time (fig 1), the change in potassium and body mass index (r = –0.31, p = 0.04), post‐race sodium and age (r = –0.3, p = 0.03), and post‐race sodium and kilometres run per week (r = 0.3, p = 0.04). These relationships suggest that the faster finishers did so with higher post‐race sodium concentrations and a smaller change in sodium over the race. Post‐race sodium was highest in the athletes with the highest training volume, and lowest in the older participants. No correlation existed between change in body weight and race time (r = 0.22, p = 0.11) or change in body weight and post‐race sodium (fig 2).
Figure 1 Relationship between post‐race serum sodium and race time.
Figure 2 The percentage change in body weight (n = 50) over the race was not associated with serum sodium after the race.
Discussion
These data suggest that ibuprofen, when taken before and during an ultradistance running race, does not significantly alter serum electrolyte concentrations. This is in contrast with previous data suggesting reduced serum sodium after prolonged exercise in subjects taking NSAIDs ad libitum.6,19,26 One main difference between the present investigation and these studies is the experimental design. Davis et al6 collected data retrospectively from subjects who presented to any of 14 emergency departments because of signs or symptoms of hyponatraemia after a marathon. Another study with a similar design found that hyponatraemia was not related to NSAID use.27 Reid et al26 examined a large cohort of marathoners during a southern hemisphere winter marathon. In that study, 13% (18/134) of the runners had chosen to take NSAIDs in the 24 h before the race. Post‐race serum creatinine, urea and potassium were raised in the runners who had taken NSAIDs. Similarly, Wharam et al19 found that ad libitum NSAID use in the 24 h before an ironman marathon increased the likelihood of lower plasma sodium after the race. None of these previous studies controlled for intake or dosage. This retrospective type of design may be misleading because, under conditions of ad libitum intake, NSAIDs may be ingested under duress or by slower participants. In fact, it has been demonstrated by this study and others26,27 that slower participants are more likely to finish races with lower blood sodium. The design in the present investigation eliminated the confounding factors that may also predispose endurance race participants to lower post‐race sodium. Experience, race time, and training levels did not differ between the ibuprofen and control groups in the present investigation.
What is already known on this topic
Few investigations of non‐steroidal anti‐inflammatory drug (NSAID) use during ultradistance endurance events have been carried out.
The few retrospective studies to date did not control for dosage or timing of NSAID intake.
Investigations using recall of ad libitum intake suggest that NSAIDs may adversely affect post‐race serum sodium concentration.
What this study adds
This is the first study to control for ibuprofen intake during a 160 km trail running race.
In subjects who ingested 1800 mg ibuprofen before and during the race, serum electrolyte concentrations after the race had not changed significantly.
As we did not demonstrate a direct effect of ibuprofen on serum electrolyte concentrations, we are unable to make assumptions about potential mechanisms. However, in situations of high AVP concentration (higher intensity exercise, syndrome of inappropriate antidiuresis, or exercise in the heat), NSAIDs may contribute to further antidiuresis and potentially the risk of hyponatraemia.6,25,31 Another difference between our study and the previous literature on NSAIDs and electrolytes is the duration of the exercise bout (marathon and ironman vs a 25 h run). It may be that higher intensity is needed for the interaction between NSAIDs and exercise for the proposed antidiuretic effect to occur. Yet we did find that NSAIDs, by themselves, in healthy subjects performing ultraendurance runs do not alter serum electrolytes.
We cannot rule out the possibility that the effects of NSAIDs on serum electrolytes depend on dose or NSAID type. The 1800 mg dose that was adhered to by the subjects in this study was chosen because it is the manufacturer's recommended daily maximal limit. In ad libitum situations, race participants may exceed this recommendation. Unfortunately dose and type of NSAID were not reported in the previous retrospective studies in this area.6,19,26,27 Ibuprofen is one type of non‐selective cyclo‐oxygenase (COX) 1 and 2 inhibitor, and aspirin and acetaminophen are other non‐selective COX‐1 and COX‐2 inhibitors. The potential differences that these, or COX‐2‐specific NSAIDs, have on electrolytes and the potential for hyponatraemia remain to be determined.
A large proportion of athletes performing ultraendurance exercise rely on NSAIDs for their analgesic effect or to reduce muscle soreness. Despite this, there is growing evidence that these analgesics may have little effect on muscle damage or soreness, and may even exacerbate the inflammatory response and endotoxaemia during exercise.18,38,40,41,42,43 Although we did not show any risk of ibuprofen on serum electrolyte concentrations in this study, there appears to be little evidence for any benefit of taking these over‐the‐counter drugs during endurance exercise.
Similarly to previous work,8,10,11,12,19,37,44 we found that lower post‐race sodium was associated with slower finishing times, older age, and lower training volume. Unlike previous work,8,10,11,12,19,34,36,37,44 we did not find a significant relationship between relative weight change and post‐race sodium concentrations even though there was an even distribution of those that gained and lost weight. This may be because we did not have any subjects who were clinically diagnosed as hyponatraemic (serum sodium <130 mmol/l) or our subject population (n = 50) was small compared with others.9,19,34,44 Although many ultraendurance athletes with low post‐race serum sodium may have retained water and thus gained weight, weight gain by itself should be only one component of a multifactorial diagnosis of hyponatraemia.
In summary, we have found that ibuprofen ingestion (1800 mg) before and during an ultradistance endurance run did not significantly affect serum electrolyte concentrations. Like others, we found that lower post‐race sodium was associated with slower finishing times, older age, and lower training volume.
Acknowledgements
This study was supported by Gatorade Sports Science and the Western States 100 Medical Board. We would like to thank the race director, Greg Soderlund, David Mack from Lodi Memorial Hospital, and Jim Heard from Sierra Nevada Memorial Hospital, without whom this research would not have been possible.
Abbreviations
AVP - arginine vasopressin
COX - cyclo‐oxygenase
NSAID - non‐steroidal anti‐inflammatory drug
Footnotes
Competing interests: None.
References
- 1.Anon Marathons: blood, sweat, and cheers. Marathon running may be extreme, but there are some lessons about exercise in it for all of us. Harv Health Lett 2003286. [PubMed] [Google Scholar]
- 2.O'Toole M L, Douglas P S, Laird R H.et al Fluid and electrolyte status in athletes receiving medical care at an ultradistance triathlon. Clin J Sport Med 19955116–122. [DOI] [PubMed] [Google Scholar]
- 3.Ayus J C, Varon J, Arieff A I. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners. Ann Intern Med. 2000 May 2 132711–714. [DOI] [PubMed] [Google Scholar]
- 4.Hew‐Butler T, Almond C, Ayus J C.et al Consensus statement of the 1st International Exercise‐Associated Hyponatremia Consensus Development Conference, Cape Town, South Africa 2005. Clin J Sport Med 200515208–213. [DOI] [PubMed] [Google Scholar]
- 5.Hiller W D. Dehydration and hyponatremia during triathlons. Med Sci Sports Exerc 198921(Suppl 5)S219–S221. [PubMed] [Google Scholar]
- 6.Davis D P, Videen J S, Marino A.et al Exercise‐associated hyponatremia in marathon runners: a two‐year experience. J Emerg Med 20012147–57. [DOI] [PubMed] [Google Scholar]
- 7.Noakes T D, Goodwin N, Rayner B L.et al Water intoxication: a possible complication during endurance exercise. Med Sci Sports Exerc 198517370–375. [PubMed] [Google Scholar]
- 8.Noakes T D, Norman R J, Buck R H.et al The incidence of hyponatremia during prolonged ultraendurance exercise. Med Sci Sports Exerc 199022165–170. [PubMed] [Google Scholar]
- 9.Noakes T D, Sharwood K, Speedy D.et al Three independent biological mechanisms cause exercise‐associated hyponatremia: evidence from 2,135 weighed competitive athletic performances. Proc Natl Acad Sci USA. 2005 20 10218550–18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speedy D B, Noakes T D, Schneider C. Exercise‐associated hyponatremia: a review. Emerg Med (Fremantle) 20011317–27. [DOI] [PubMed] [Google Scholar]
- 11.Speedy D B, Noakes T D, Rogers I R.et al Hyponatremia in ultradistance triathletes. Med Sci Sports Exerc 199931809–815. [DOI] [PubMed] [Google Scholar]
- 12.Speedy D B, Rogers I R, Noakes T D.et al Exercise‐induced hyponatremia in ultradistance triathletes is caused by inappropriate fluid retention. Clin J Sport Med 200010272–278. [DOI] [PubMed] [Google Scholar]
- 13.Vrijens D M, Rehrer N J. Sodium‐free fluid ingestion decreases plasma sodium during exercise in the heat. J Appl Physiol 1999861847–1851. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong L E, Curtis W C, Hubbard R W.et al Symptomatic hyponatremia during prolonged exercise in heat. Med Sci Sports Exerc 199325543–549. [PubMed] [Google Scholar]
- 15.Dugas J P, Noakes T D. Hyponatraemic encephalopathy despite a modest rate of fluid intake during a 109 km cycle race. Br J Sports Med. 2005;39: e38;discussion e38, [DOI] [PMC free article] [PubMed]
- 16.Galun E, Tur‐Kaspa I, Assia E.et al Hyponatremia induced by exercise: a 24‐hour endurance march study. Miner Electrolyte Metab 199117315–320. [PubMed] [Google Scholar]
- 17.Nieman D C, Dumke C L, Henson D A.et al Muscle damage is linked to cytokine changes following a 160‐km race. Brain Behav Immun 200519398–403. [DOI] [PubMed] [Google Scholar]
- 18.Smetanka R D, Lambert G P, Murray R.et al Intestinal permeability in runners in the 1996 Chicago marathon. Int J Sport Nutr 19999426–433. [DOI] [PubMed] [Google Scholar]
- 19.Wharam P C, Speedy D B, Noakes T D.et al NSAID use increases the risk of developing hyponatremia during an Ironman triathlon. Med Sci Sports Exerc 200638618–622. [DOI] [PubMed] [Google Scholar]
- 20.Blum M, Aviram A. Ibuprofen induced hyponatraemia. Rheumatol Rehabil 198019258–259. [DOI] [PubMed] [Google Scholar]
- 21.Bush T M, Shlotzhauer T L, Imai K. Nonsteroidal anti‐inflammatory drugs. Proposed guidelines for monitoring toxicity. West J Med 199115539–42. [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia E B, Ruitenberg A, Madretsma G S.et al Hyponatraemic coma induced by desmopressin and ibuprofen in a woman with von Willebrand's disease. Haemophilia 20039232–234. [DOI] [PubMed] [Google Scholar]
- 23.Goodenough G K, Lutz L J. Hyponatremic hypervolemia caused by a drug: drug interaction mistaken for syndrome of inappropriate ADH. J Am Geriatr Soc 198836285–286. [DOI] [PubMed] [Google Scholar]
- 24.Rault R M. Case report: hyponatremia associated with nonsteroidal antiinflammatory drugs. Am J Med Sci 1993305318–320. [DOI] [PubMed] [Google Scholar]
- 25.Petersson I, Nilsson G, Hansson B G.et al Water intoxication associated with non‐steroidal anti‐inflammatory drug therapy. Acta Med Scand 1987221221–223. [DOI] [PubMed] [Google Scholar]
- 26.Reid S A, Speedy D B, Thompson J M.et al Study of hematological and biochemical parameters in runners completing a standard marathon. Clin J Sport Med 200414344–353. [DOI] [PubMed] [Google Scholar]
- 27.Hew T D, Chorley J N, Cianca J C.et al The incidence, risk factors, and clinical manifestations of hyponatremia in marathon runners. Clin J Sport Med 20031341–47. [DOI] [PubMed] [Google Scholar]
- 28.Stichtenoth D O, Wagner B, Frolich J C. Effect of selective inhibition of the inducible cyclooxygenase on renin release in healthy volunteers. J Investig Med 199846290–296. [PubMed] [Google Scholar]
- 29.Murray M D, Brater D C. Renal toxicity of the nonsteroidal anti‐inflammatory drugs. Annu Rev Pharmacol Toxicol 199333435–465. [DOI] [PubMed] [Google Scholar]
- 30.Lee A, Cooper M G, Craig J C.et al The effects of nonsteroidal anti‐inflammatory drugs (NSAIDs) on postoperative renal function: a meta‐analysis. Anaesth Intensive Care 199927574–580. [DOI] [PubMed] [Google Scholar]
- 31.Wen S F. Nephrotoxicities of nonsteroidal anti‐inflammatory drugs. J Formos Med Assoc 199796157–171. [PubMed] [Google Scholar]
- 32.Verbalis J G. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab 200317471–503. [DOI] [PubMed] [Google Scholar]
- 33.Stoff J S, Rosa R M, Silva P.et al Indomethacin impairs water diuresis in the DI rat: role of prostaglandins independent of ADH. Am J Physiol 1981241F231–F237. [DOI] [PubMed] [Google Scholar]
- 34.Almond C S, Shin A Y, Fortescue E B.et al Hyponatremia among runners in the Boston Marathon. N Engl J Med 20053521550–1556. [DOI] [PubMed] [Google Scholar]
- 35.Irving R A, Noakes T D, Buck R.et al Evaluation of renal function and fluid homeostasis during recovery from exercise‐induced hyponatremia. J Appl Physiol 199170342–348. [DOI] [PubMed] [Google Scholar]
- 36.Frizzell R T, Lang G H, Lowance D C.et al Hyponatremia and ultramarathon running. JAMA 1986255772–774. [PubMed] [Google Scholar]
- 37.Noakes T. Hyponatremia in distance runners: fluid and sodium balance during exercise. Curr Sports Med Rep 20021197–207. [DOI] [PubMed] [Google Scholar]
- 38.Nieman D C, Henson D A, Dumke C L.et al Ibuprofen use, endotoxemia, inflammation, and plasma cytokines during ultramarathon competition. Brain Behav Immun 200620578–584. [DOI] [PubMed] [Google Scholar]
- 39.Dill D B, Costill D L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 197437247–248. [DOI] [PubMed] [Google Scholar]
- 40.Peterson J M, Trappe T A, Mylona E.et al Ibuprofen and acetaminophen: effect on muscle inflammation after eccentric exercise. Med Sci Sports Exerc 200335892–896. [DOI] [PubMed] [Google Scholar]
- 41.Hasson S M, Daniels J C, Divine J G.et al Effect of ibuprofen use on muscle soreness, damage, and performance: a preliminary investigation. Med Sci Sports Exerc 1993259–17. [DOI] [PubMed] [Google Scholar]
- 42.Donnelly A E, Maughan R J, Whiting P H. Effects of ibuprofen on exercise‐induced muscle soreness and indices of muscle damage. Br J Sports Med 199024191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan A J, Chang R T, Gisolfi C V. Gastrointestinal permeability following aspirin intake and prolonged running. Med Sci Sports Exerc 199628698–705. [DOI] [PubMed] [Google Scholar]
- 44.Sharwood K, Collins M, Goedecke J.et al Weight changes, sodium levels, and performance in the South African Ironman Triathlon. Clin J Sport Med 200212391–399. [DOI] [PubMed] [Google Scholar]