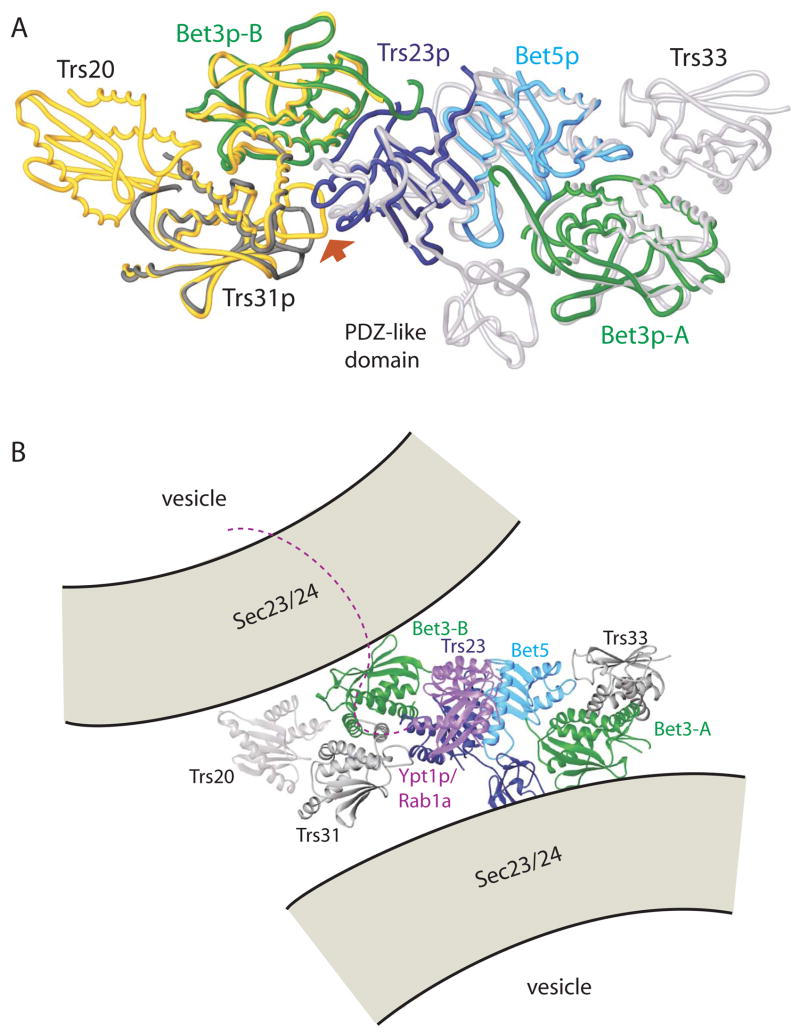
Figure 5.
A superposition of the yeast TRAPPI subcomplex containing Trs31p/Bet3p-B/Trs23p/Bet5p/Bet3p-A with the Trs20/Trs31/Bet3-B (yellow, PDB ID 2J3W) and Trs23/Bet5/Bet3-A/Trs33 (white, 2J32) complexes from vertebrates. A PDZ-like domain is present only in mammalian Trs23. Yeast proteins are colored as in Figure 1, (A) and (C), and the complex is oriented as in panels A–C, left. The largest deviations between the yeast and vertebrate assemblies are at the 3-way interface between Trs23p, Trs31p, and Bet3p-B (arrow). (B) Model for the mammalian TRAPP core as it tethers two COPII coated vesicles via interactions between Bet3 subunits of TRAPP and Sec23 of the COPII coat. The two copies of Bet3 and the two vesicles are related by the same 2-fold rotation about an axis perpendicular to the plane of the page. Equivalent surface patches on Bet3-A and –B are accessible to both vesicles only if TRAPP is oriented as shown. The C-terminal residues (175–206) absent from Ypt1p (purple) in our structure could span the ~40 Å to the membrane. Ypt1p/Rab1a becomes anchored in the membrane by its prenylated C-terminus.