Abstract
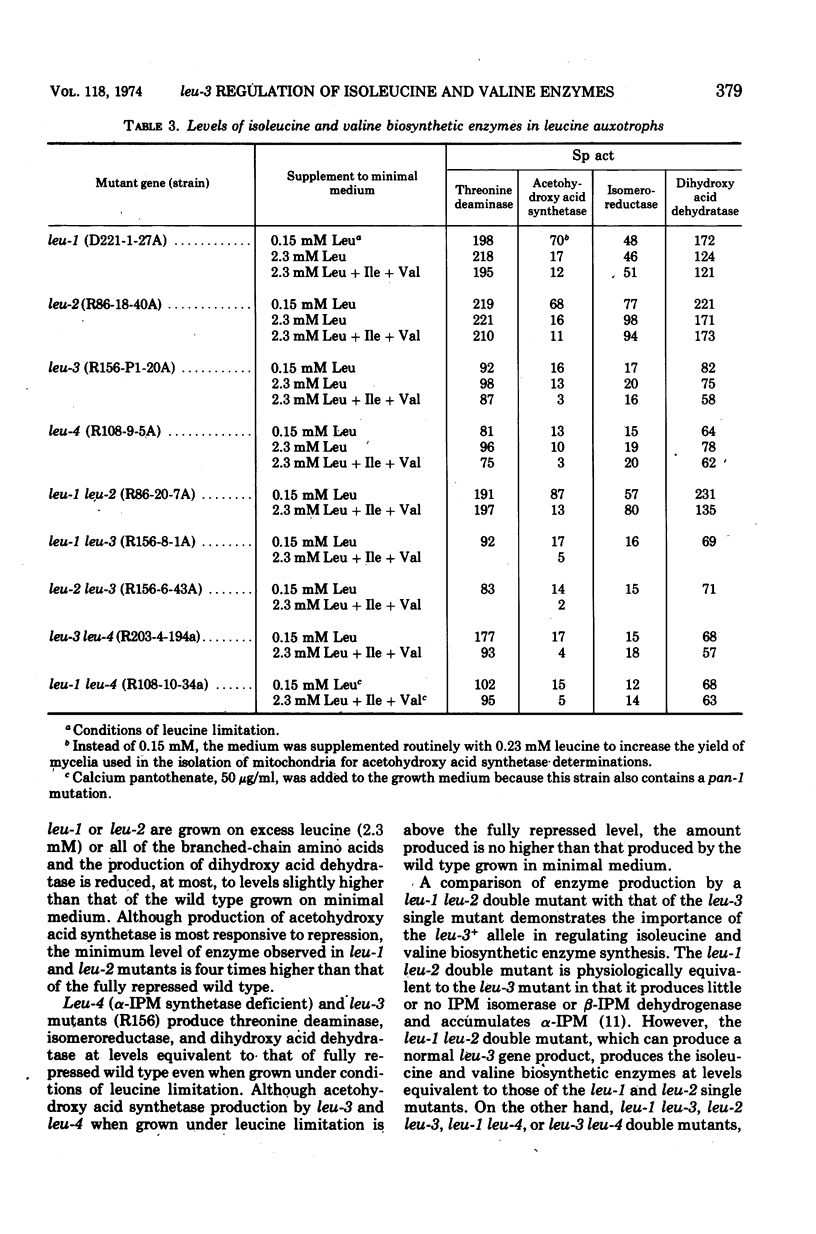
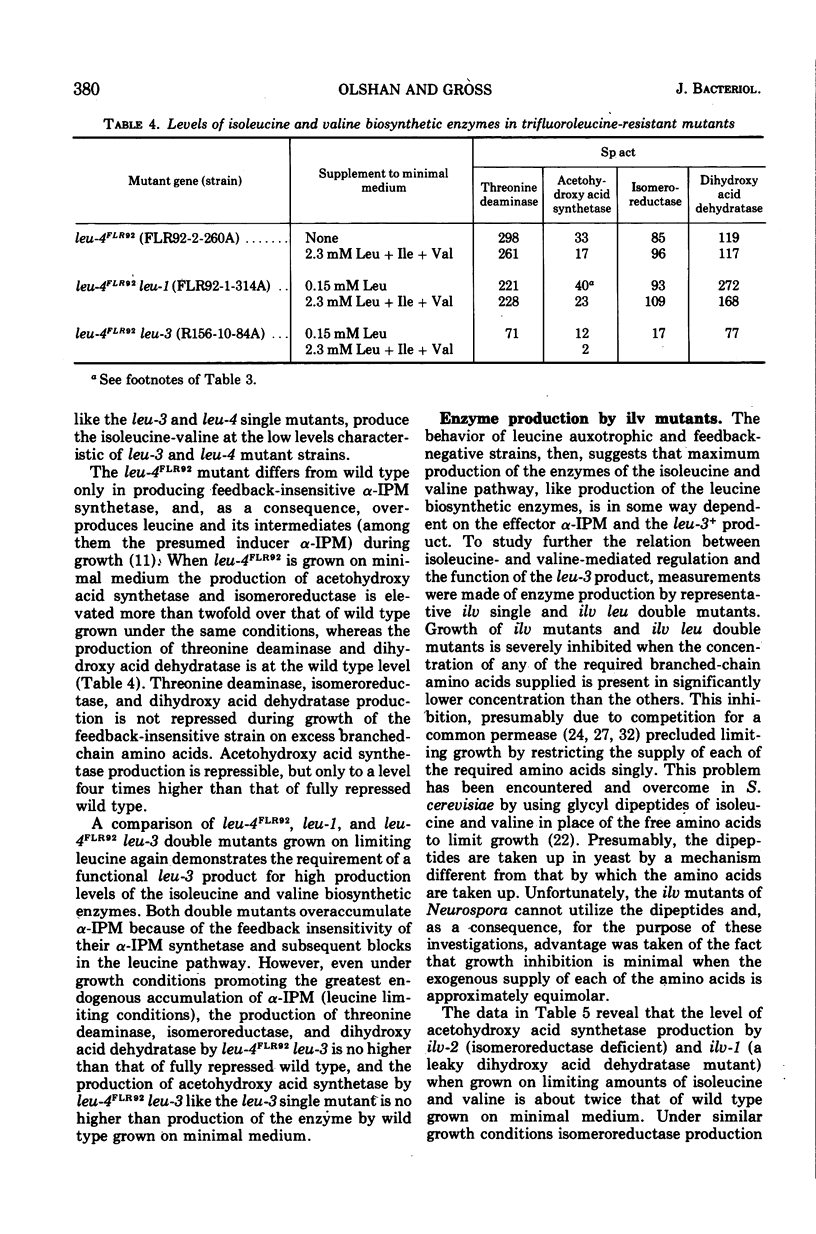
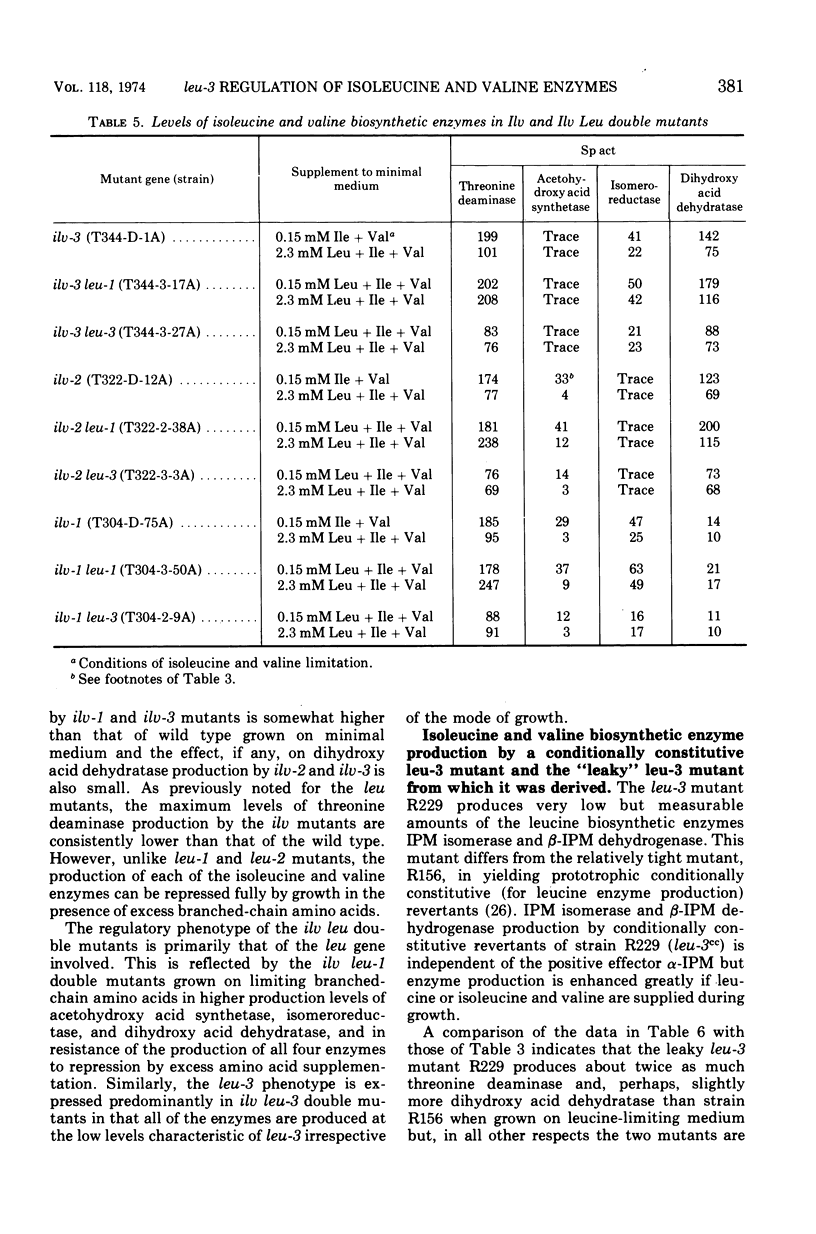
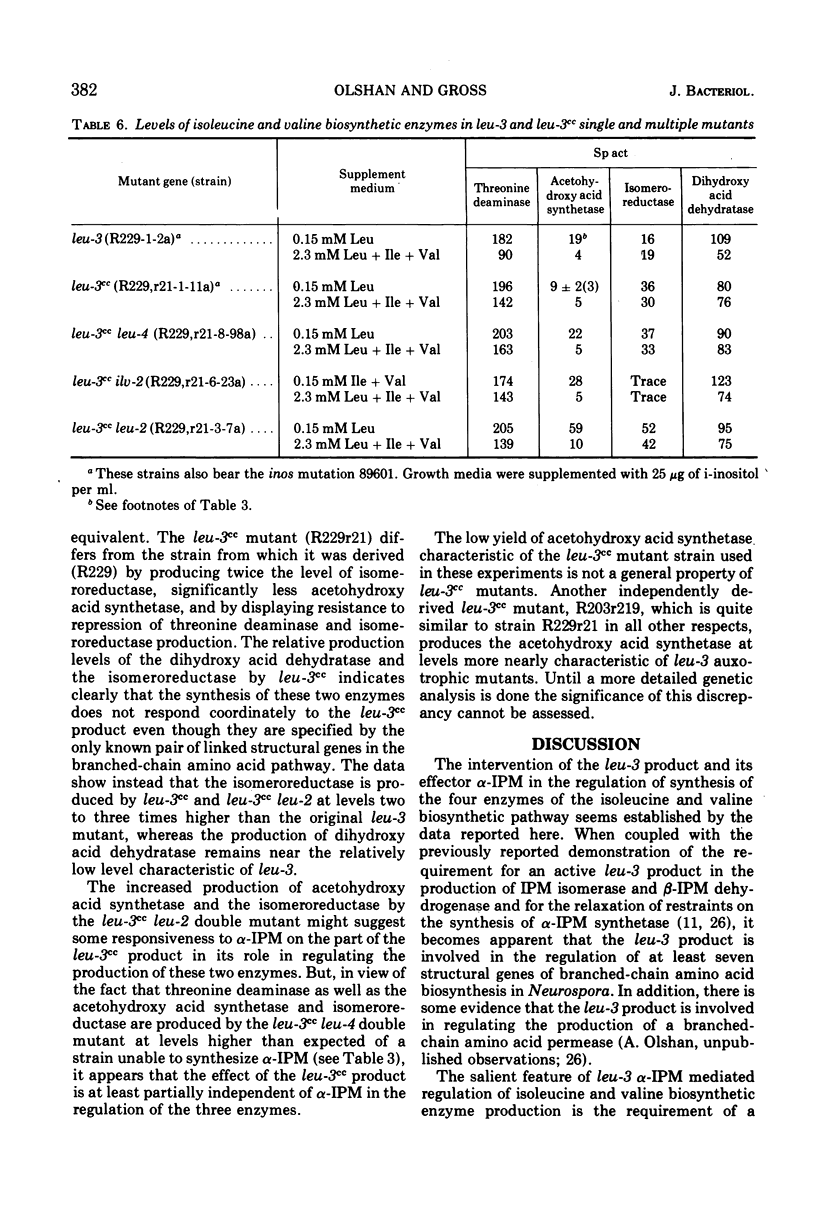
The production by Neurospora of the enzymes of isoleucine and valine synthesis in response to specific end product-derived signals depends upon the presence of an effective leu-3 regulatory product and its effector α-isopropylmalate (α-IPM). In leu-3+ strains, threonine deaminase production is repressed as a function of available isoleucine, acetohydroxy acid synthetase as a function of valine, and the isomeroreductase and dihydroxy acid dehydratase as a function of isoleucine and leucine. In the absence of an effective leu-3 regulatory product, α-isopropylmalate, or both, the production of isoleucine and valine biosynthetic enzymes is fixed at or near fully repressed levels even under conditions of severe end product limitation. Thus, in addition to its involvement in the regulation of expression of the three structural genes of leucine synthesis, the leu-3 α-IPM regulatory product is necessary for full expression of at least four genes specifying the structure of the enzymes of isoleucine and valine synthesis. It is suggested that the leu-3 α-IPM regulatory element may facilitate transcription of the genetically dispersed cistrons either by imposing specificity on ribonucleic acid polymerase for structurally similar promoters adjacent to each of the cistrons or by “opening” promoters after interaction with nearly identical stretches of deoxyribonucleic acid near each of the structural genes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmiller D. H. Neurospora mutants with mitochondria deficient in dihydroxy acid dehydratase. Properties of dihydroxy acid dehydratase from mutant strain 332. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1000–1006. doi: 10.1016/0006-291x(72)90311-7. [DOI] [PubMed] [Google Scholar]
- Altmiller D. H., Wagner R. P. Deficiency of dihydroxy acid dehydratase in the mitochondria of the iv-I mutants of Neurospora crassa. Biochem Genet. 1970 Apr;4(2):243–251. doi: 10.1007/BF00485775. [DOI] [PubMed] [Google Scholar]
- Altmiller D. H., Wagner R. P. Purification and properties of dihydroxy acid dehydratase from soluble and mitochondrial fractions of Neurospora crassa. Arch Biochem Biophys. 1970 May;138(1):160–170. doi: 10.1016/0003-9861(70)90295-x. [DOI] [PubMed] [Google Scholar]
- Beckwith J., Grodzicker T., Arditti R. Evidence for two sites in the lac promoter region. J Mol Biol. 1972 Aug 14;69(1):155–160. doi: 10.1016/0022-2836(72)90031-9. [DOI] [PubMed] [Google Scholar]
- Blatt J. M., Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XX. Multiple forms of acetohydroxy acid synthetase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):444–450. doi: 10.1016/s0006-291x(72)80071-8. [DOI] [PubMed] [Google Scholar]
- Caroline D. F., Harding R. W., Kuwana H., Satyanarayana T., Wagner R. P. The iv-3 mutants of Neurospora crassa. II. Activity of acetohydroxy acid synthetase. Genetics. 1969 Jul;62(3):487–494. doi: 10.1093/genetics/62.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer L., Eakin E., Wagner R. P. Acetohydroxy acid synthetase with a pH optimum of 7.5 from Neurospora crassa mitochondria: characterization and partial purification. J Bacteriol. 1972 Oct;112(1):453–464. doi: 10.1128/jb.112.1.453-464.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. R. The regulation of synthesis of leucine biosynthetic enzymes in Neurospora. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1538–1546. doi: 10.1073/pnas.54.6.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R. W., Caroline D. F., Wagner R. P. The pyruvate dehydrogenase complex from the mitochondrial fraction of Neurospora crassa. Arch Biochem Biophys. 1970 Jun;138(2):653–661. doi: 10.1016/0003-9861(70)90393-0. [DOI] [PubMed] [Google Scholar]
- Holzer H., Cennamo C., Boll M. Product activation of yeast threonine dehydratase by ammonia. Biochem Biophys Res Commun. 1964;14:487–492. doi: 10.1016/0006-291x(64)90256-6. [DOI] [PubMed] [Google Scholar]
- Kashmiri S. V., Gross S. R. Mutations affecting the regulation of production of the enzymes of leucine synthesis in Neurospora. Genetics. 1970 Mar-Apr;64(3):423–440. [PMC free article] [PubMed] [Google Scholar]
- Kiritani K., Narise S., Wagner R. P. The dihydroxy acid dehydratase of Neurospora crassa. J Biol Chem. 1966 May 10;241(9):2042–2046. [PubMed] [Google Scholar]
- Kuwana H., Caroline D. F., Harding R. W., Wagner R. P. An acetohydroxy acid synthetase from Neurospora crassa. Arch Biochem Biophys. 1968 Oct;128(1):184–193. doi: 10.1016/0003-9861(68)90021-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leiter E. H., LaBrie D. A., Bergquist A., Wagner R. P. In vitro mitochondrial complementation in Neurospora crassa. Biochem Genet. 1971 Dec;5(6):549–561. doi: 10.1007/BF00485673. [DOI] [PubMed] [Google Scholar]
- Levinthal M., Williams L. S., Umbarger H. E. Role of threonine deaminase in the regulation of isoleucine and valine biosynthesis. Nat New Biol. 1973 Nov 21;246(151):65–68. doi: 10.1038/newbio246065a0. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Hereford L. M. Multivalent repression of isoleucine- valine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jun;98(3):857–862. doi: 10.1128/jb.98.3.857-862.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 3. Properties and regulation of the activity of acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):507–511. doi: 10.1111/j.1432-1033.1967.tb19560.x. [DOI] [PubMed] [Google Scholar]
- O'Neill J. P., Freundlich M. Two forms of biosynthetic acetohydroxy acid synthetase in Salmonella typhimurium. Biochem Biophys Res Commun. 1972 Jul 25;48(2):437–443. doi: 10.1016/s0006-291x(72)80070-6. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. I. Properties of two amino acid transport systems. Biochim Biophys Acta. 1969 Jan 28;173(1):113–127. doi: 10.1016/0005-2736(69)90042-x. [DOI] [PubMed] [Google Scholar]
- Paul J. General theory of chromosome structure and gene activation in eukaryotes. Nature. 1972 Aug 25;238(5365):444–446. doi: 10.1038/238444a0. [DOI] [PubMed] [Google Scholar]
- Polacco J. C., Gross S. R. The product of the leu-3 cistron as a regulatory element for the production of the leucine biosynthetic enzymes of Neurospora. Genetics. 1973 Jul;74(3):443–459. doi: 10.1093/genetics/74.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler D. R. Genetic control of the uptake of amino acids in Neurospora. Genetics. 1966 Aug;54(2):677–685. doi: 10.1093/genetics/54.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Positive control of transcription by a bacteriophage sigma factor. Nature. 1970 Mar 14;225(5237):1009–1012. doi: 10.1038/2251009a0. [DOI] [PubMed] [Google Scholar]
- Wagner R. P., Somers C. E., Bergquist A. GENE STRUCTURE AND FUNCTION IN NEUROSPORA. Proc Natl Acad Sci U S A. 1960 May;46(5):708–717. doi: 10.1073/pnas.46.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley W. R., Matchett W. H. Tryptophan transport in Neurospora crassa. I. Specificity and kinetics. J Bacteriol. 1966 Dec;92(6):1698–1705. doi: 10.1128/jb.92.6.1698-1705.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


