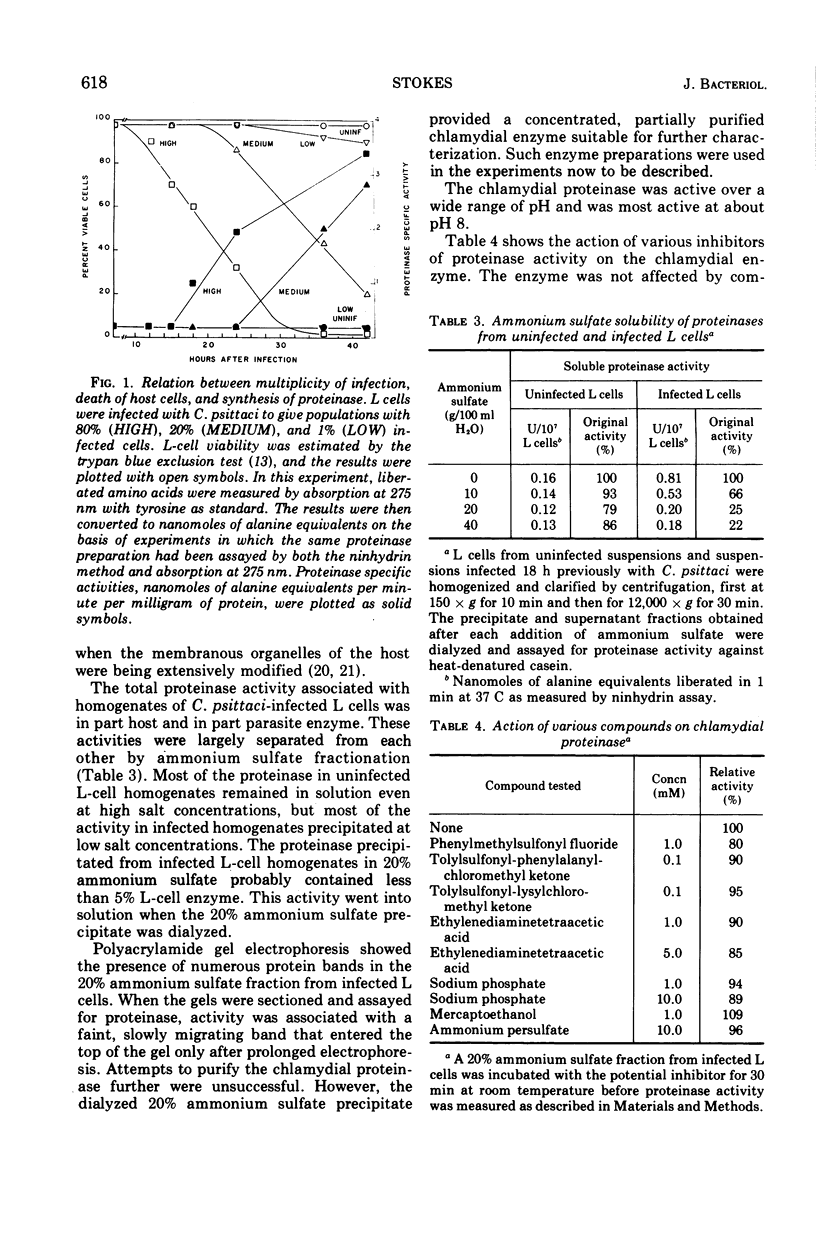
Abstract
L cells (mouse fibroblasts) infected with Chlamydia psittaci (strain meningopneumonitis) produced a proteinase differing in solubility in ammonium sulfate from the proteinase of uninfected L cells. Synthesis of the enzyme was inhibited by chloramphenicol but not by cycloheximide, indicating that the new proteinase in infected L cells was synthesized by Chlamydia psittaci. The chlamydial proteinase had no demonstrable ion requirements and was not inhibited by a variety of inhibitors of proteinase activity. Gel filtration experiments suggested a molecular weight of approximately 250,000. The proteinase appeared in infected L cells at the time host cells began to die and the large chlamydial cells began to reorganize into small ones. Some possible functions for the chlamydial proteinase were proposed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Effect of infection with the meningopneumonitis agent on deoxyribonucleic acid and protein synthesis by its L-cell host. J Bacteriol. 1969 Feb;97(2):653–657. doi: 10.1128/jb.97.2.653-657.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordová N., Wilt J. C. Lysosomes and the "toxicity" of Rickettsiales. I. Cytochemical studies of macrophages inoculated in vitro with C. psittaci 6BC. Can J Microbiol. 1972 Apr;18(4):457–464. doi: 10.1139/m72-071. [DOI] [PubMed] [Google Scholar]
- Kordová N., Wilt J. C., Sadiq M. Lysosomes in L cells infected with Chlamydia psittaci 6BC strain. Can J Microbiol. 1971 Jul;17(7):955–959. doi: 10.1139/m71-152. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin H. S. Inhibition of thymidine kinase activity and deoxyribonucleic acid synthesis in L cells infected with the meningopneumonitis agent. J Bacteriol. 1968 Dec;96(6):2054–2065. doi: 10.1128/jb.96.6.2054-2065.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCONN J. D., TSURU D., YASUNOBU K. T. BACILLUS SUBTILIS NEUTRAL PROTEINASE. I. A ZINC ENZYME OF HIGH SPECIFIC ACTIVITY. J Biol Chem. 1964 Nov;239:3706–3715. [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron Microscopic Observations on the Fine Structure of Cell Walls of Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1332–1337. doi: 10.1128/jb.104.3.1332-1337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLIMANS W. F., DAVIS E. V., GLOVER F. L., RAKE G. W. The submerged culture of mammalian cells; the spinner culture. J Immunol. 1957 Nov;79(5):428–433. [PubMed] [Google Scholar]
- Millet J., Acher R., Aubert J. P. Biochemical and physiological properties of an extracellular protease produced by Bacillus megaterium. Biotechnol Bioeng. 1969 Nov;11(6):1233–1246. doi: 10.1002/bit.260110617. [DOI] [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. The contribution of model systems to the understanding of infectious diseases. Perspect Biol Med. 1971 Spring;14(3):486–502. doi: 10.1353/pbm.1971.0024. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Uriel J. Isolation and some propeties of a proteolytic enzyme from Escherichia coli (protease I). Eur J Biochem. 1971 Dec 10;23(3):435–442. doi: 10.1111/j.1432-1033.1971.tb01638.x. [DOI] [PubMed] [Google Scholar]
- SQUIRE P. G. A RELATIONSHIP BETWEEN THE MOLECULAR WEIGHTS OF MACROMOLECULES AND THEIR ELUTION VOLUMES BASED ON A MODEL FOR SEPHADEX GEL FILTRATION. Arch Biochem Biophys. 1964 Sep;107:471–478. doi: 10.1016/0003-9861(64)90303-0. [DOI] [PubMed] [Google Scholar]
- Stokes G. V. Cycloheximide-resistant glycosylation in L cells infected with Chlamydia psittaci. Infect Immun. 1974 Mar;9(3):497–499. doi: 10.1128/iai.9.3.497-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. Formation and destruction of internal membranes in L cells infected with Chlamydia psittaci. Infect Immun. 1973 Feb;7(2):173–177. doi: 10.1128/iai.7.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Effect of penicillin on the multiplication of meningopneumonitis organisms (Chlamydia psittaci). J Bacteriol. 1968 Oct;96(4):875–880. doi: 10.1128/jb.96.4.875-880.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Friis R. R., Moulder J. W. Effect of chloramphenicol, rifampicin, and nalidixic acid on Chlamydia psittaci growing in L cells. J Infect Dis. 1973 Feb;127(2):155–163. doi: 10.1093/infdis/127.2.155. [DOI] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Availability of bases and nucleosides as precursors of nucleic acids in L cells and in the agent of meningopneumonitis. J Bacteriol. 1966 Jun;91(6):2362–2367. doi: 10.1128/jb.91.6.2362-2367.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Inhibition of Deoxyribonucleic Acid Synthesis in Synchronized Populations of L Cells Infected with Chlamydia psittaci. Infect Immun. 1971 Feb;3(2):363–364. doi: 10.1128/iai.3.2.363-364.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


