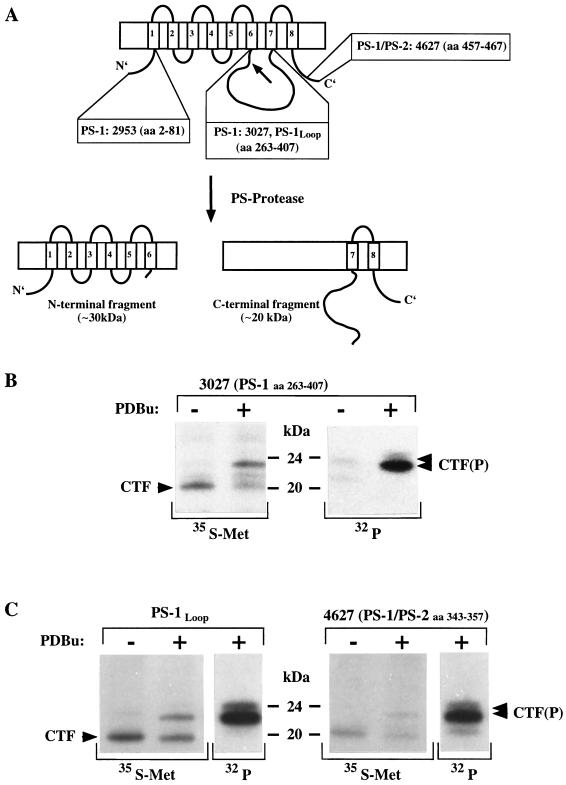
Figure 1.
(A) Schematic drawing showing the potential eight-trans-membrane domain structure (32) and the proteolytic processing of the PS proteins (30). The proteolytic cleavage (indicated by an arrow) occurs within the large hydrophilic loop between TM6 and TM7 generating an ≈30-kDa N-terminal fragment and an ≈20-kDa CTF (30). The epitopes of the antibodies used are indicated. (B) PDBu stimulates phosphorylation of the CTF of PS-1. Untransfected COS-7 cells were radiolabeled in the presence (+) or absence (−) of PDBu with [35S]methionine (35S-Met) as well as [32P]orthophosphate (32P). Cell lysates were immunoprecipitated with antibody 3027. After [35S]methionine labeling a major polypeptide of ≈20 kDa (CTF) was observed under control conditions. Upon PKC stimulation with PDBu the ≈20-kDa band shifted to ≈23 kDa. Labeling with [32P]orthophosphate revealed two weakly phosphorylated polypeptides of ≈22 kDa and ≈23 kDa under control conditions. PDBu treatment resulted in the detection of a highly phosphorylated polypeptide of ≈23 kDa and a weaker band of ≈24 kDa [CTF(P)]. (C) Results very similar to B were obtained with two independent antibodies. Untransfected COS-7 cells were radiolabeled in the presence (+) or absence (−) of PDBu with [35S]methionine (35S-Met) as well as [32P]orthophosphate (32P). Cell lysates were immunoprecipitated with antibodies BOS 4627 (26) and PS-1loop (30).