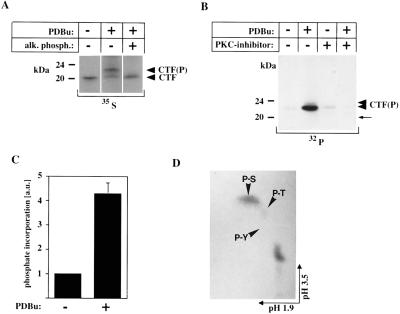
Figure 2.
(A) Dephosphorylation reverses the molecular mass shift of the PS-1 CTF. Immunoprecipitates from PDBu-treated COS-7 cells labeled with [35S]methionine were incubated in the presence or absence of alkaline phosphatase. Phosphatase treatment induces a shift of the phosphorylated 23-kDa CTF observed after PDBu treatment back to 20 kDa, which comigrates with the unphosphorylated CTF from unstimulated cells. (B) Inhibition of PKC suppresses phosphorylation of the 20-kDa PS-CTF. Untransfected COS-7 cells were radiolabeled in the presence (+) or absence (−) of PDBu with or without (±) the PKC-specific inhibitor GF109203X with [32P]orthophosphate. Cell lysates were immunoprecipitated with antibody 3027. The phosphorylated ≈23-kDa and ≈24-kDa polypeptides are marked by arrowheads [CTF(P)]; the position of the unphosphorylated CTF, which is not visible by 32P labeling is marked by an arrow. This band was detected by parallel labeling with [35S]methionine (data not shown). (C) Quantification of PKC-mediated phosphorylation of the PS-1 CTF. Untransfected COS-7 cells were radiolabeled in the presence (+) or absence (−) of PDBu with [32P]orthophosphate (32P). Cell lysates were immunoprecipitated with antibody 3027 and 32P incorporation was quantified by phosphorimaging (34). (Bars = ± SE; n = 3.) (D) Phosphoamino acid analysis of PKC-phosphorylated CTF. Untransfected COS-7 cells were radiolabeled in the presence of PDBu with [32P]orthophosphate. The CTF was isolated by immunoprecipitation using antibody 3027. The position of phosphoserine (P-S), phosphothreonine (P-T), and phosphotyrosine (P-Y) is indicated.