Abstract
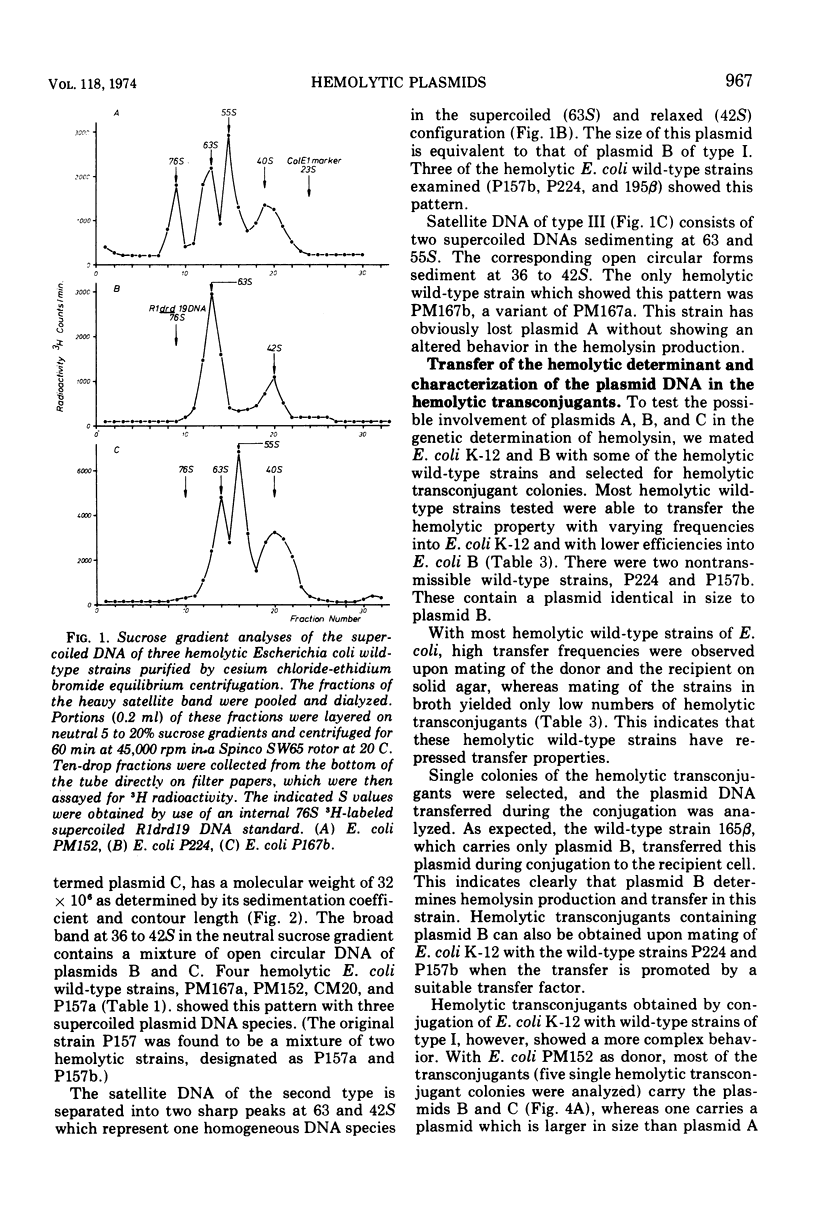
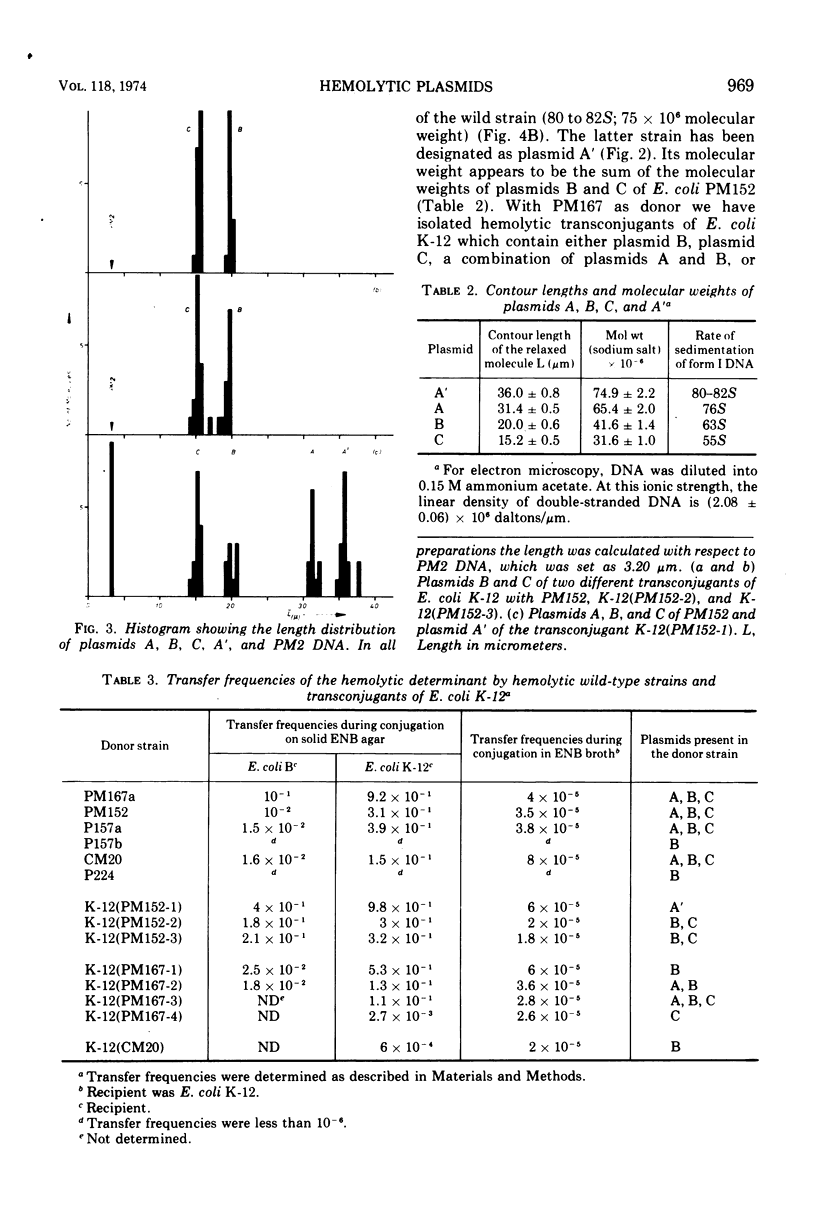
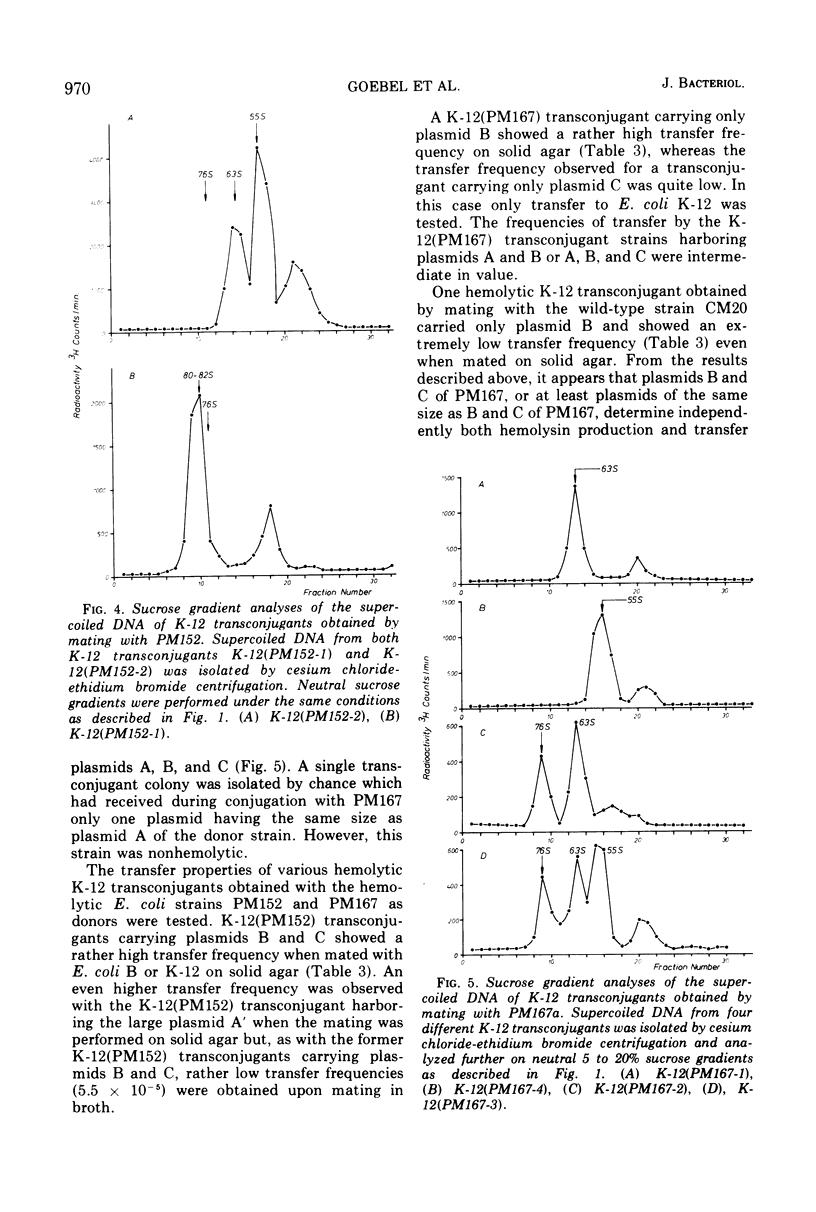
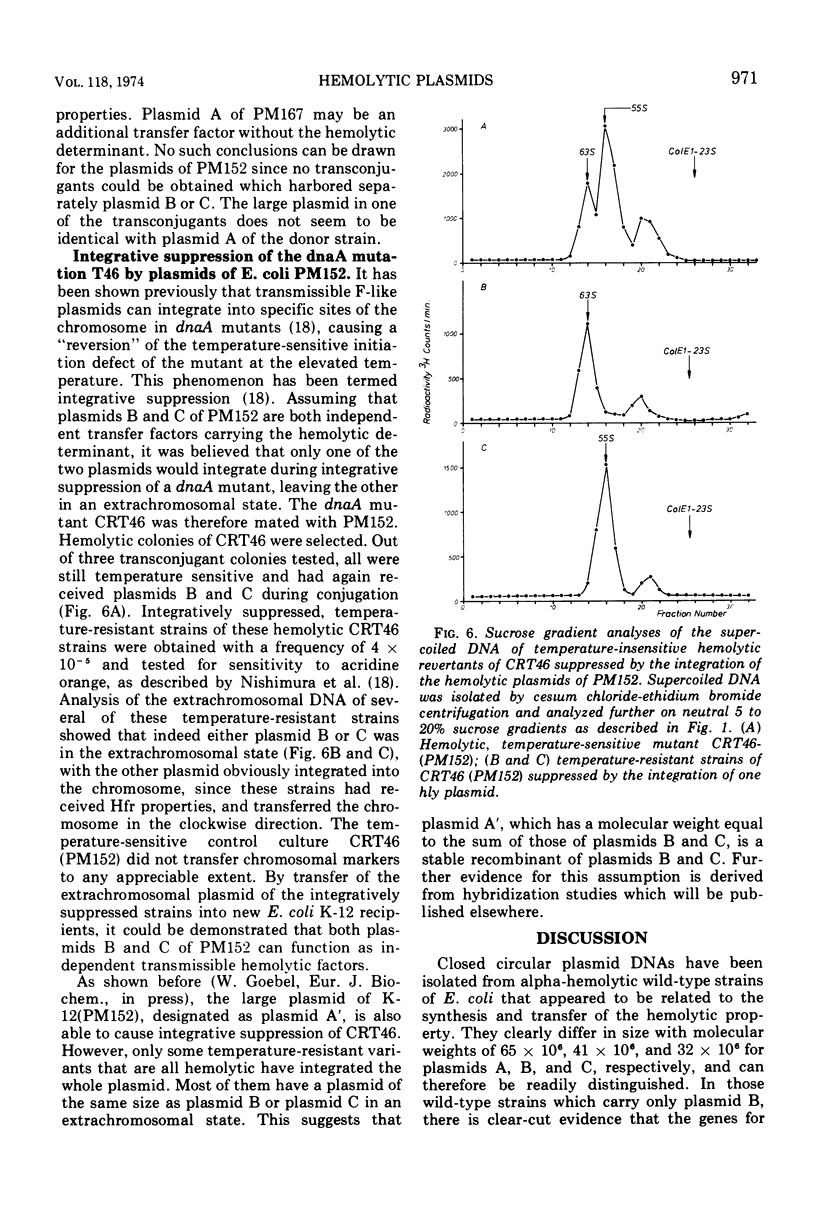
Covalently closed extrachromosomal deoxyribonucleic acid (DNA) was isolated from alpha-hemolytic wild-type strains of Escherichia coli. Most strains examined were able to transfer the hemolytic property with varying frequencies to nonhemolytic recipient strains. Out of eight naturally isolated alphahemolytic E. coli strains, four contained a set of three different supercoiled DNAs with sedimentation coefficients of 76S (plasmid A), 63S (plasmid B), and 55S (plasmid C). The sedimentation coefficients and the contour lengths of the isolated molecules correspond to molecular weights of 65 × 106, 41 × 106, and 32 × 106. Three alpha-hemolytic wild-type strains carried only one plasmid with a molecular weight of 41 × 106, and one strain harbored two plasmids with molecular weights of 41 × 106 and 32 × 106. Alpha-hemolytic transconjugants were obtained by conjugation of E. coli K-12 with the hemolytic wild-type strains. A detailed examination revealed that plasmids with the same sizes as plasmids B and C of the wild-type strains can be transferred separately or together to the recipients. Both plasmids possess the hemolytic determinant and transfer properties. Plasmid A appears to be, at least in one wild-type strain, an additional transfer factor without a hemolytic determinant. In one case a hemolytic factor was isolated, after conjugation, that is larger in size than plasmid A and appears to be a recombinant of both plasmids B and C.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S., Lewis M. J. Characterization of a transfer factor associated with drug resistance in Salmonella typhimurium. Nature. 1965 Nov 27;208(5013):843–849. doi: 10.1038/208843a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Lewis M. J. Drug resistance and its transfer in Salmonella typhimurium. Nature. 1965 May 8;206(984):579–583. doi: 10.1038/206579a0. [DOI] [PubMed] [Google Scholar]
- Bak A. L., Christiansen G., Christiansen C., Stenderup A., Orskov I., Orskov F. Circular DNA molecules controlling synthesis and transfer of the surface antigen (K88) in Escherichia coli. J Gen Microbiol. 1972 Nov;73(2):373–385. doi: 10.1099/00221287-73-2-373. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bujard H. Electron microscopy of single-stranded DNA. J Mol Biol. 1970 Apr 14;49(1):125–137. doi: 10.1016/0022-2836(70)90381-5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. E. Existence of the colicinogenic factor-sex factor ColI-b-P9 as a supercoiled circular DNA-protein relaxation complex. Biochem Biophys Res Commun. 1970 Oct 9;41(1):150–156. doi: 10.1016/0006-291x(70)90481-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Goebel W., Helinski D. R. Nicking activity of an endonuclease. I. Transfer ribonucleic acid complex of Escherichia coli. Biochemistry. 1970 Nov 24;9(24):4793–4801. doi: 10.1021/bi00826a025. [DOI] [PubMed] [Google Scholar]
- Goebel W. Studies on extrachromosomal DNA elements. Replication of the colicinogenic factor Col E1 in two temperature sensitive mutants of Escherichia coli defective in DNA replication. Eur J Biochem. 1970 Aug;15(2):311–320. doi: 10.1111/j.1432-1033.1970.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Filamentous phages specific for the I sex factor. Nature. 1968 Mar 23;217(5134):1184–1186. doi: 10.1038/2171184a0. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The transmissible nature of the genetic factor in Escherichia coli that controls haemolysin production. J Gen Microbiol. 1967 Apr;47(1):153–161. doi: 10.1099/00221287-47-1-153. [DOI] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Birch-Andersen A. Episome-carried surface antigen K88 of Escherichia coli. 3. Morphology. J Bacteriol. 1967 Feb;93(2):740–748. doi: 10.1128/jb.93.2.740-748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae IV. Interactions between resistance transfer factor and F-factor in Escherichia coli K-12. J Bacteriol. 1962 Apr;83:727–735. doi: 10.1128/jb.83.4.727-735.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]




