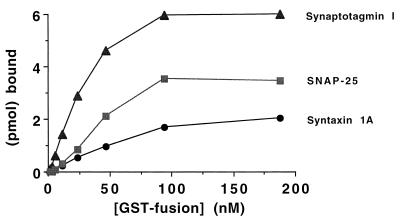
Figure 2.
Measurement of synprint interactions in a solid-phase immunoassay. Stoichiometric interaction of the synprint peptide with syntaxin 1A, SNAP-25, and syt I. Picomoles of specific, bound GST-syntaxin 1A (•), GST-syt I (80–421) (▴), or GST-SNAP-25 (▪) are plotted as a function of the concentration of GST-fusion protein added. Microtiter plates were coated with 6.4 pmol of purified LII–III(718–963) (corrected for adsorption efficiency) followed by incubation with 2-fold serial dilutions of purified GST-syntaxin 1A, GST-syt I (80–421), GST-SNAP-25, or a GST control protein, and detection with a mouse IgG-2b anti-GST mAb and an anti-mouse IgG-2b-specific mAb conjugated to alkaline phosphatase. Absorbance readings were converted to molar concentrations of bound GST-fusion protein via a reference curve relating A410 to pmol of the anti-mouse IgG-2b-specific alkaline phosphatase conjugated mAb. Stoichiometries are calculated as the molar ratio of bound GST-fusion protein to adsorbed LII–III(718–963).