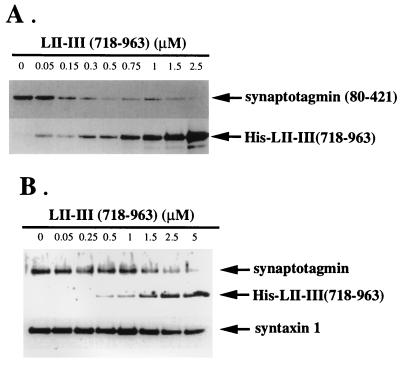
Figure 3.
Displacement of synaptotagmin binding to syntaxin by the synprint peptide. (A) Synprint peptide competes with the binding of recombinant syt I to syntaxin 1A. GST-syntaxin 1A bound to glutathione-Sepharose beads was incubated with a constant amount of the purified recombinant cytoplasmic domain (residues 80–421) of syt I and increasing amounts of His-LII–III(718–963) as indicated, in a binding buffer containing 20 μM of free Ca2+. Beads were washed, and bound proteins were eluted with 15 mM reduced glutathione in 50 mM of Tris⋅HCl (pH 8), and analyzed by immunoblotting with 1D12, a mAb against the carboxyl terminals of both syt I and syt II, and T7.Tag antibody as described. (B) The synprint peptide competes for the interaction of native synaptotagmin with syntaxin. GST-VAMP/synaptobrevin 2 bound to glutathione-Sepharose beads was incubated with a constant amount of solubilized synaptosomes and an increasing amount of His-LII–III(718–963) as indicated, in a binding buffer with 20 μM free Ca2+. Syntaxin and SNAP-25 from solubilized synaptosomes were associated with GST-VAMP/synaptobrevin 2 bound to glutathione-Sepharose as described (35) to form an SDS-resistant complex, which serves as an acceptor for binding either recombinant LII–III(718–963) or native synaptotagmin from solubilized synaptosomes. After extensive washing with incubation buffer, GST-VAMP/synaptobrevin 2-bound protein complexes were eluted with reduced glutathione and analyzed by immunoblotting with T7.Tag, 1D12, and 10H5, a syntaxin 1 antibody. Each lane contains an equivalent amount of GST-syntaxin 1A (A) and GST-VAMP/synaptobrevin 2 (B), bound to beads, as confirmed by anti-GST immunoblotting (data not shown).