Abstract
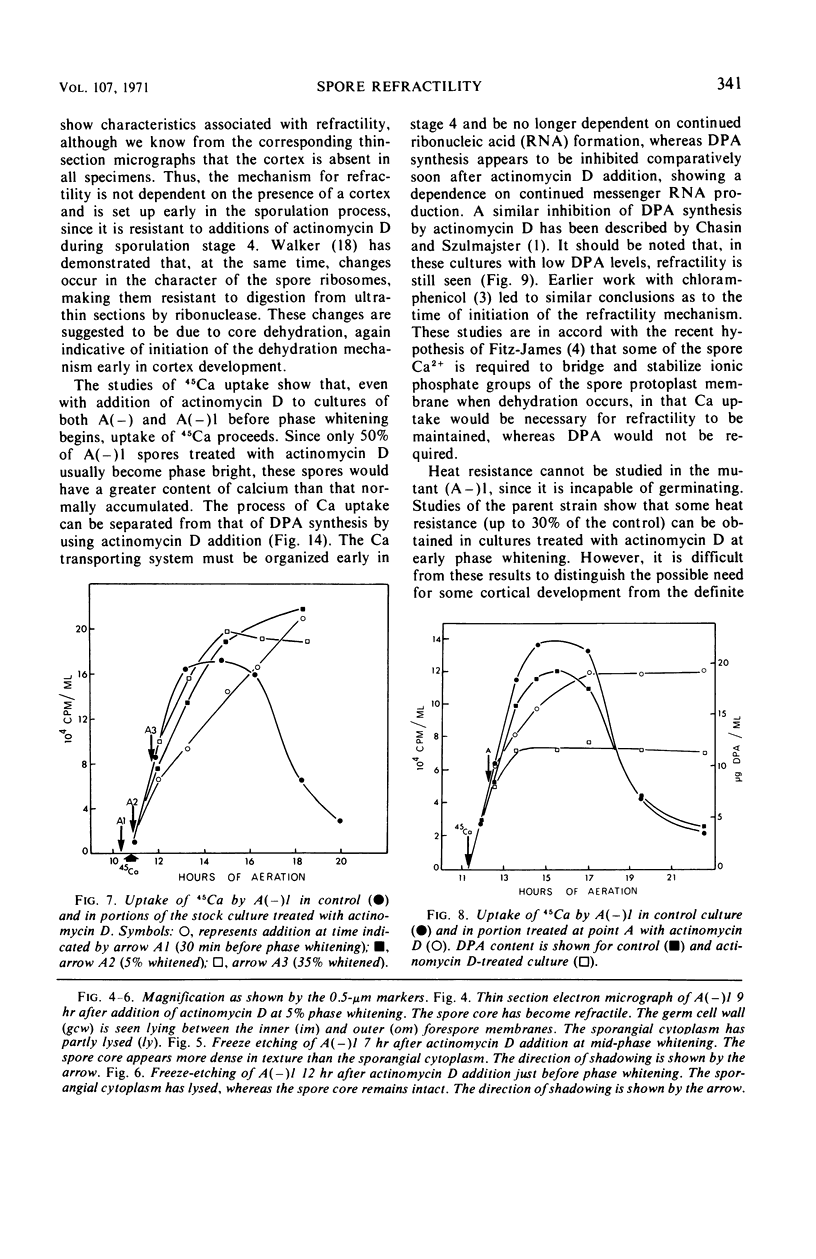
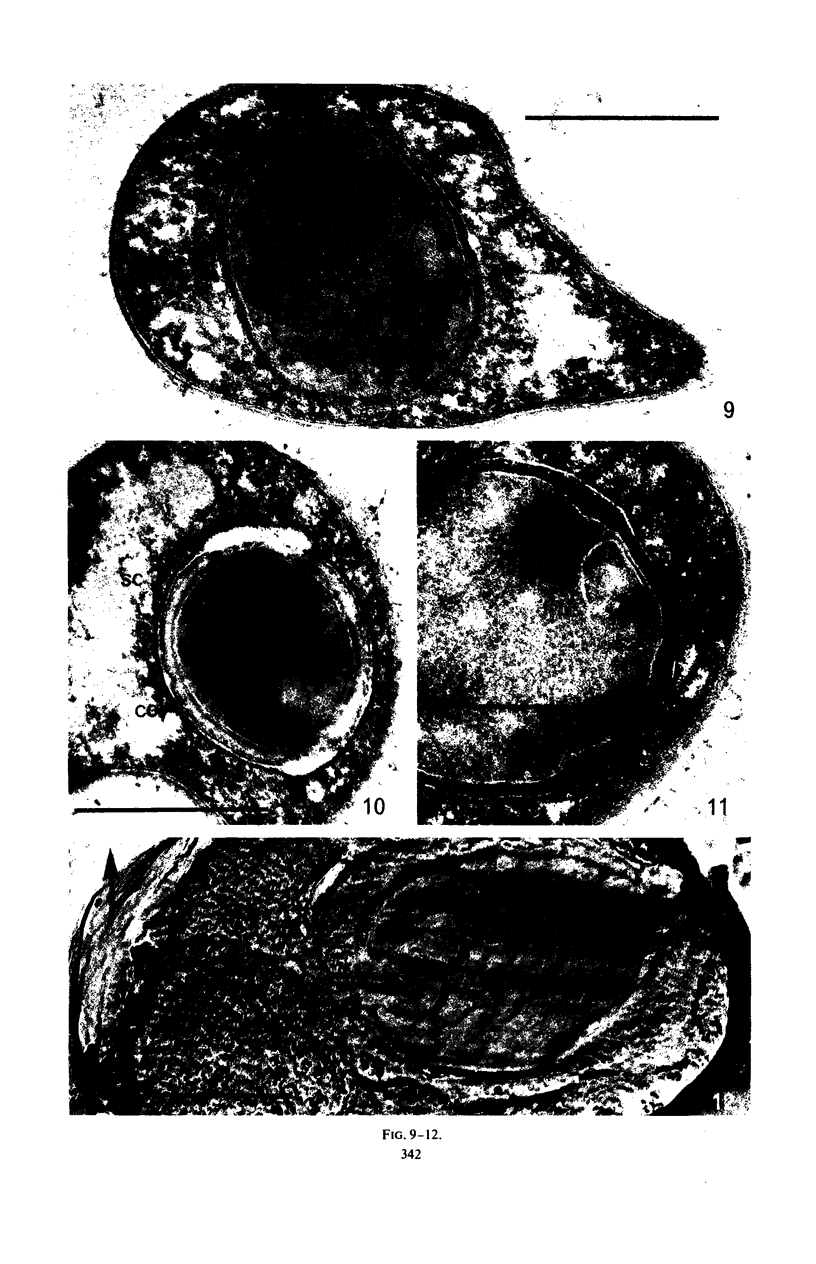
Refractility as indicated by light microscopy, electron microscopy of thin sections, and freeze fracture etching was increased and maintained in a cortexless mutant, A(−)1, of Bacillus cereus var. alesti by the addition during sporulation stage 4 of actinomycin D, which prevents the terminal lysis of spore core associated with sporulation in this organism. 45Calcium uptake levels and dipicolinic acid (DPA) content were similarly maintained. The location of these components appears to be in the spore protoplast. In the parent A(−), treated with actinomycin D during stage 4, spore particles with similar morphology to the mutant, that is without a cortex and with the characteristics of refractility, were obtained. A major difference in sensitivity to actinomycin D between the processes of 45Ca uptake and DPA synthesis was observed. Some heat resistance in A(−) made cortexless by actinomycin D could be observed. These studies indicate that the role of the cortex is not to produce the dehydrated refractile spore state but to maintain it.
Full text
PDF


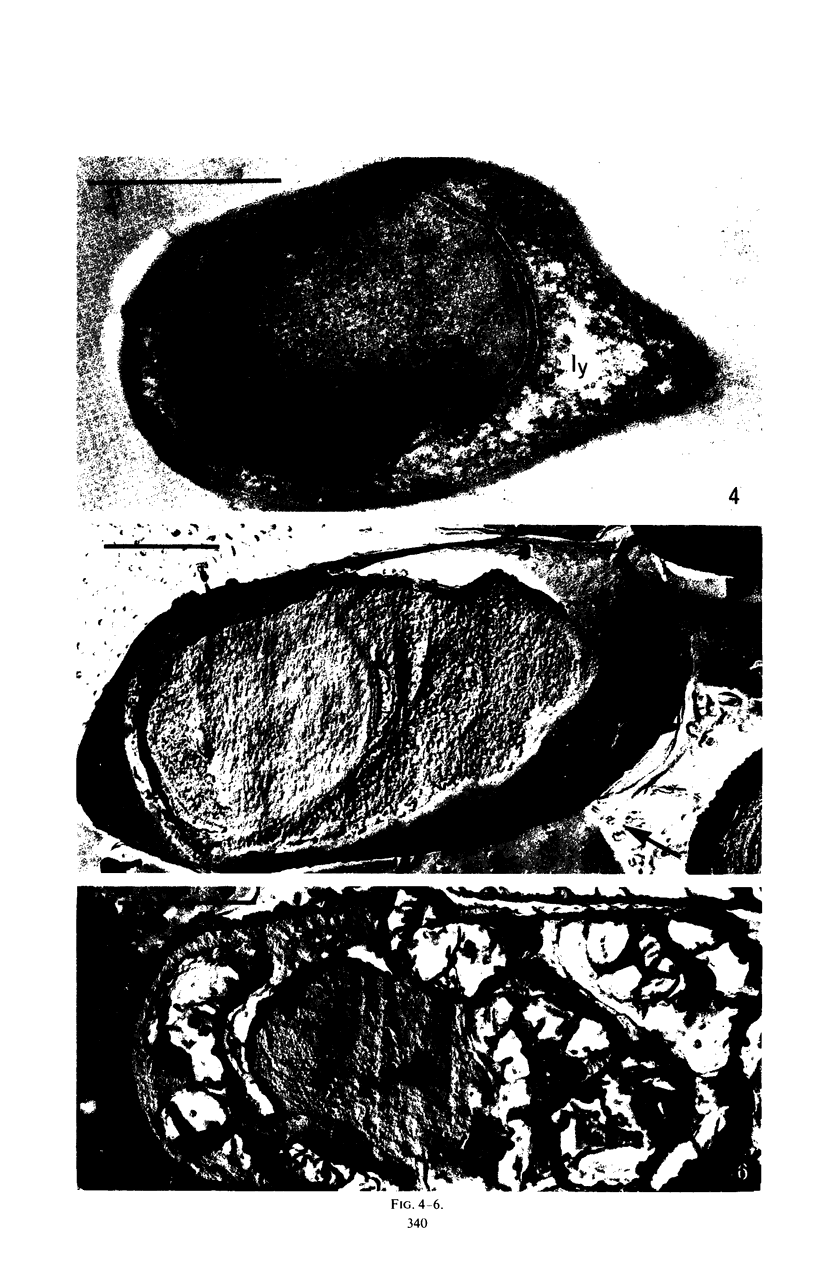

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donnellan J. E., Jr, Setlow R. B. Thymine Photoproducts but not Thymine Dimers Found in Ultraviolet-Irradiated Bacterial Spores. Science. 1965 Jul 16;149(3681):308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. C., YOUNG I. E. Comparison of species and yarieties of the genus Bacillus. Structure and nucleic acid content of spores. J Bacteriol. 1959 Dec;78:743–754. doi: 10.1128/jb.78.6.743-754.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HITCHINS A. D., GOULD G. W. RELEASE OF CORES FROM BACTERIAL SPORES BY MECHANICAL BREAKAGE IN ACIDIC MEDIA. Nature. 1964 Aug 22;203:895–896. doi: 10.1038/203895b0. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. C., Snell N. S., Burr H. K. Water Permeability of Bacterial Spores and the Concept of a Contractile Cortex. Science. 1960 Aug 26;132(3426):544–545. doi: 10.1126/science.132.3426.544. [DOI] [PubMed] [Google Scholar]
- Pearce S. M., Fitz-James P. C. Sporulation of a cortexless mutant of a variant of Bacillus cereus. J Bacteriol. 1971 Jan;105(1):339–348. doi: 10.1128/jb.105.1.339-348.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinter V., Stastná J. Spores of microorganisms. XXI. Conversion of outgrowing spores of Bacillus cereus to refractile forms by basic peptides and proteins. Folia Microbiol (Praha) 1967;12(3):301–307. doi: 10.1007/BF02868748. [DOI] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D. The location of chemical components on ultrathin sections of Bacillus cereus embedded in glycol methacrylate. J Appl Bacteriol. 1969 Dec;32(4):463–467. doi: 10.1111/j.1365-2672.1969.tb00999.x. [DOI] [PubMed] [Google Scholar]
- YOUNG I. E. A relationship between the free amino acid pool, dipicolinic acid, calcium from resting spores of Bacillus megaterium. Can J Microbiol. 1959 Apr;5(2):197–202. doi: 10.1139/m59-024. [DOI] [PubMed] [Google Scholar]
- YOUNG I. E., FITZ-JAMES P. C. Chemical and morphological studies of bacterial spore formation. II. Spore and parasporal protein formation in Bacillus cereus var. alesti. J Biophys Biochem Cytol. 1959 Dec;6:483–498. doi: 10.1083/jcb.6.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E., JAMES P. C. Chemical and morphological studies of bacterial spore formation. IV. The development of spore refractility. J Cell Biol. 1962 Jan;12:115–133. doi: 10.1083/jcb.12.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]