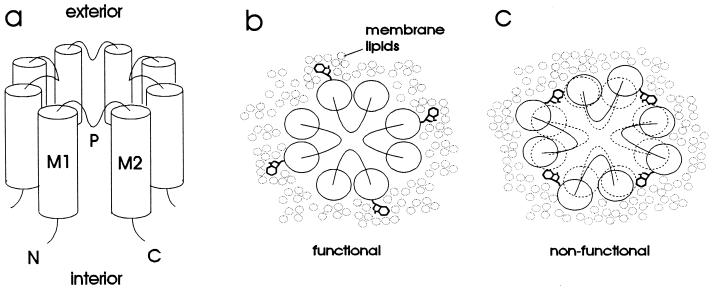
Figure 1.
(a) Schematic diagram of proposed inward rectifier structure. The channel is comprised of four subunits, each of which has two transmembrane α helices (M1 and M2) and a pore region (P) that loops part of the way into the transmembrane portion of the protein. Both the amino (N) and carboxyl (C) termini are inside the cell. (b and c) Rationale for tryptophan-scanning mutagenesis, illustrated on a schematic view of the channel from the extracellular side. (b) Substitution of tryptophan into a lipid-exposed position is accommodated by the membrane lipids and therefore does not disrupt the structure of the channel. (c) Substitution of tryptophan into an internal position disrupts the protein structure and therefore renders the channel nonfunctional.