Abstract
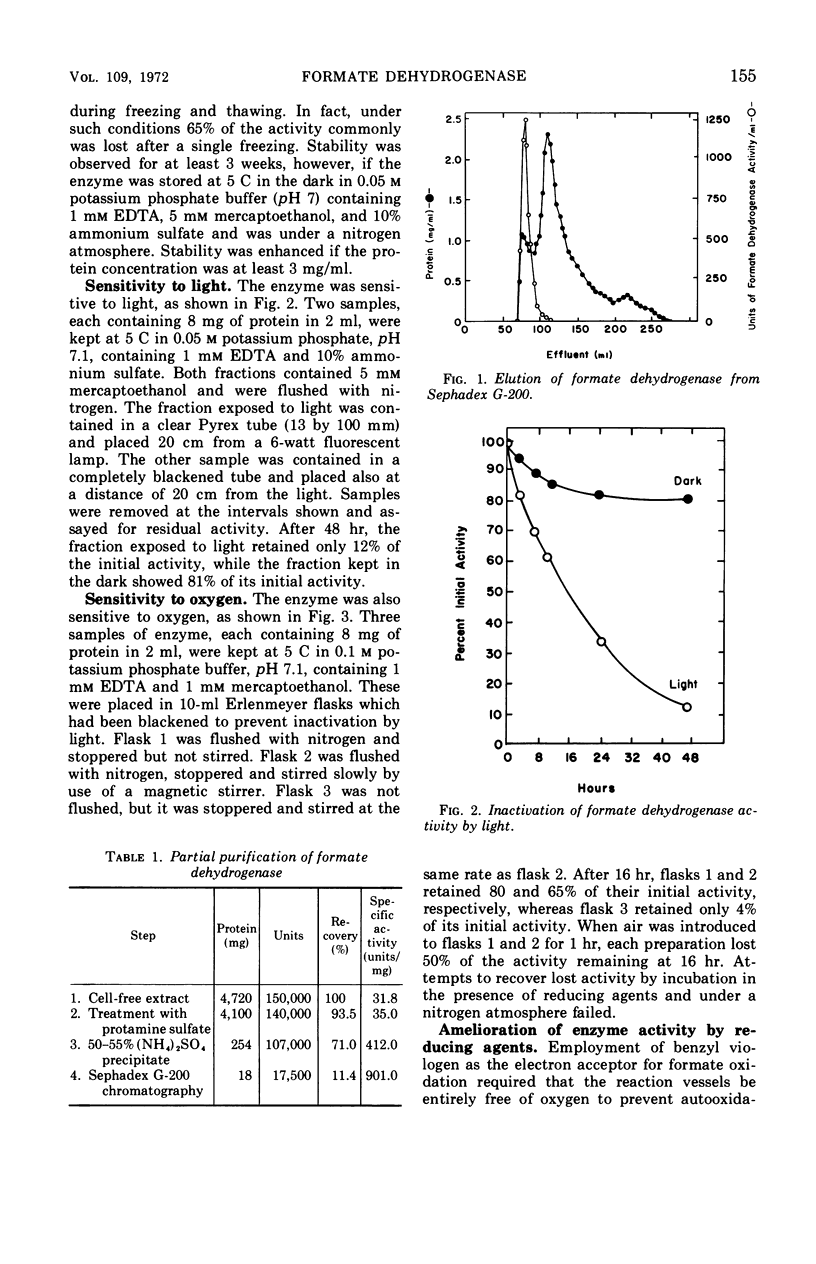
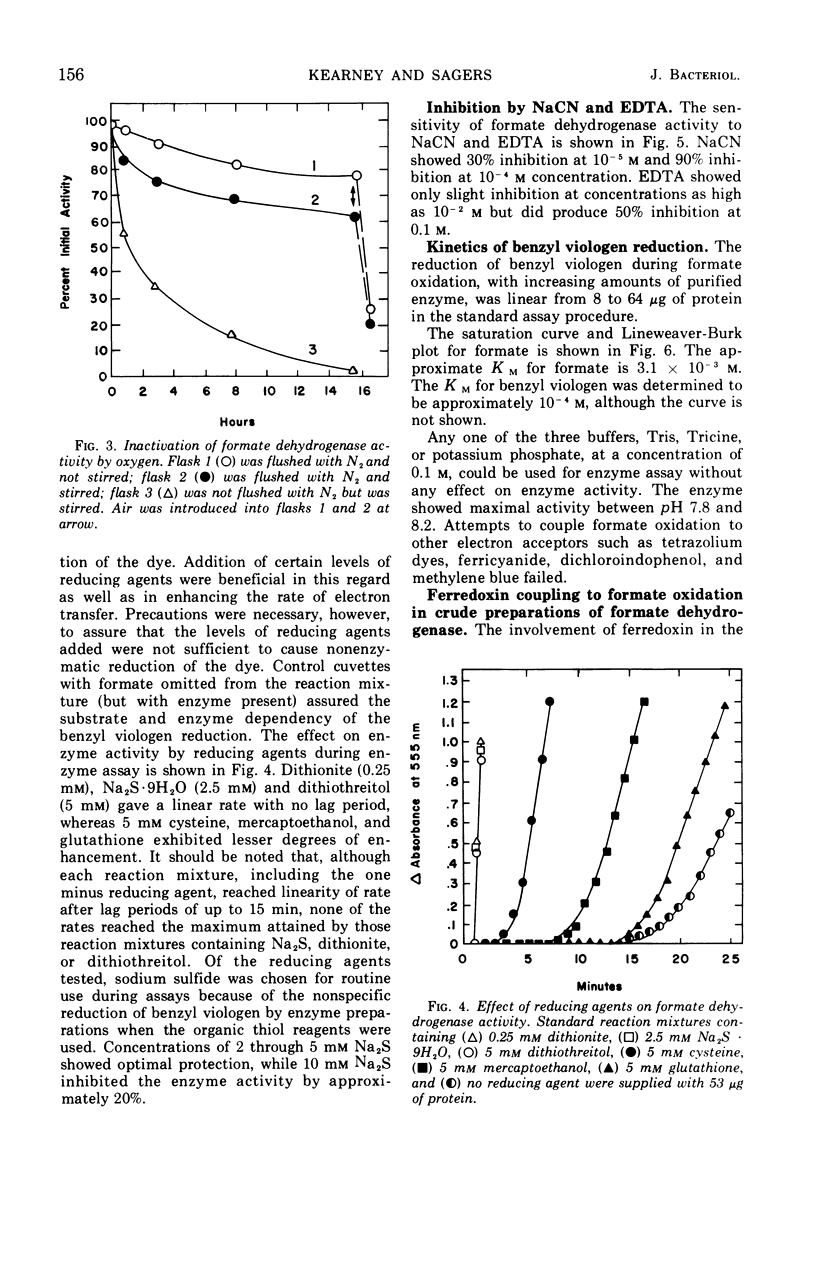
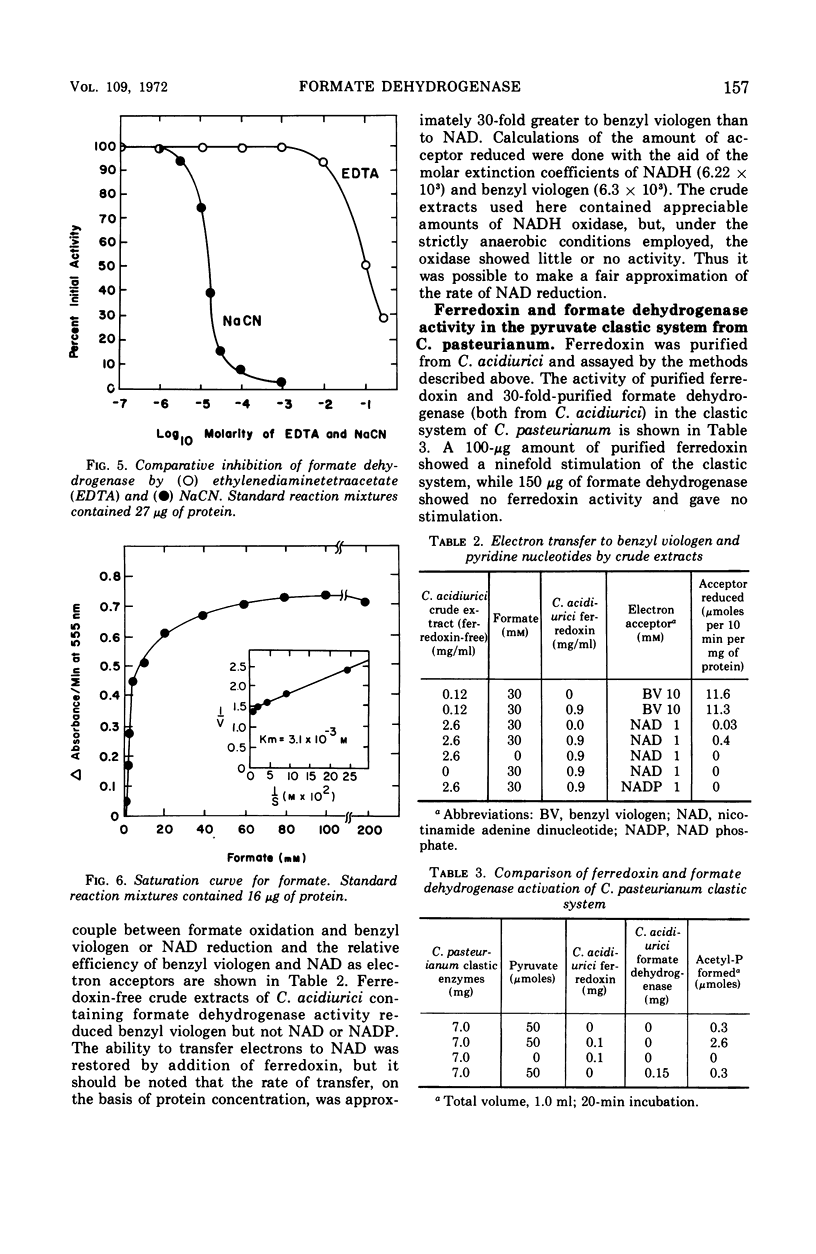
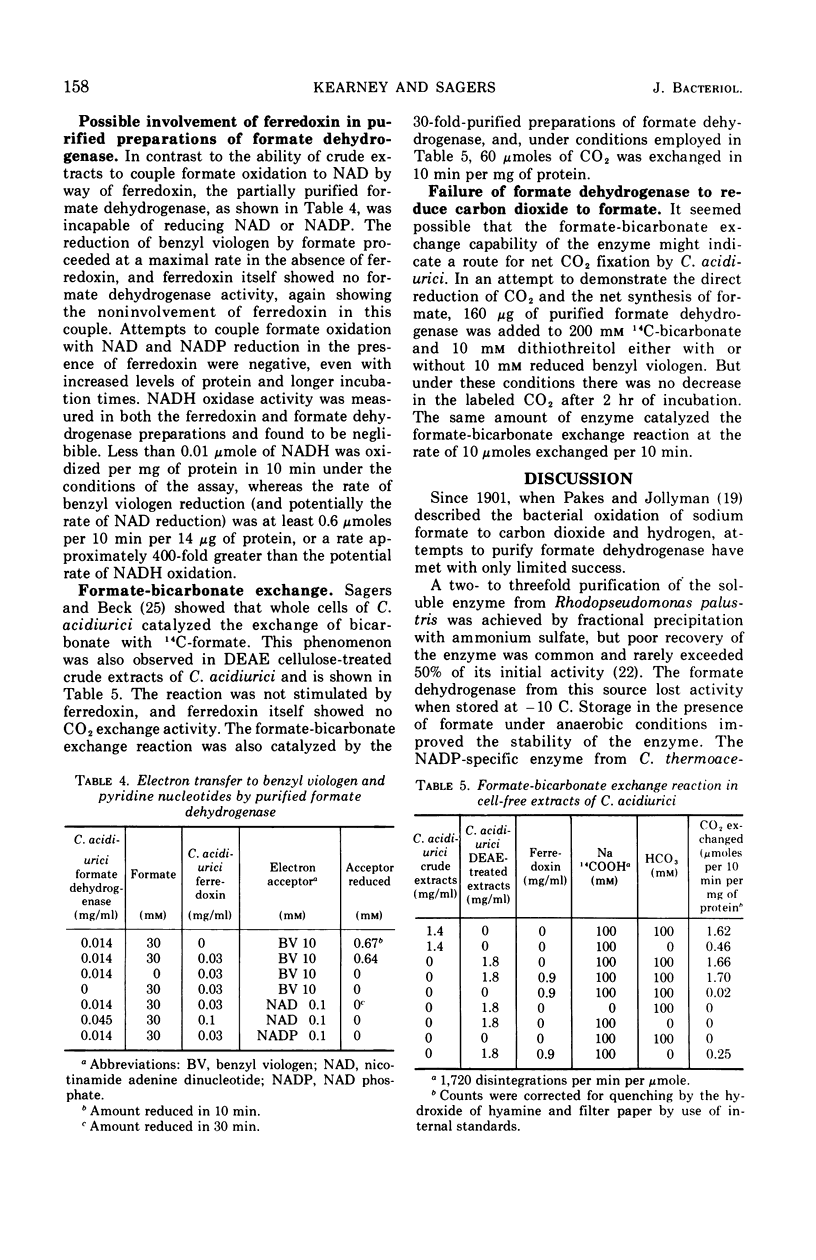
Partial purification of formate dehydrogenase from Clostridium acidiurici has been accomplished, and some properties of the enzyme have been determined. The molecular weight of the protein is at least 200,000 daltons. The enzyme showed marked instability to freezing and thawing and was inhibited strongly by oxygen and by light. Such inhibition was not reversed by incubation in the presence of thiol compounds. Cyanide inhibited the enzyme 90% at 0.1 mm concentrations, but ethylenediaminetetraacetate produced only slight inhibition at concentrations as high as 50 mm. The purified enzyme showed no ferredoxin activity in the Clostridium pasteurianum clastic system during pyruvate oxidation. Crude preparations of the enzyme could be coupled through ferredoxin to the reduction of nicotinamide adenine dinucleotide during formate oxidation, but the purified enzyme could not catalyze the reduction of pyridine nucleotides by formate in the presence of ferredoxin. Formate oxidation with the purified enzyme was readily coupled to benzyl viologen reduction, in which case ferredoxin was not required. An exchange between formate and bicarbonate was catalyzed by both crude and purified preparations of the enzyme, but the net synthesis of formate from CO2 was not accomplished.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECK J. V., SAGERS R. D. Studies on the formation of formate, glycine, serine, pyruvate and acetate from purines by Clostridium acidi-urici. J Bacteriol. 1956 Aug;72(2):199–208. doi: 10.1128/jb.72.2.199-208.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRILL W. J., WOLIN E. A., WOLFE R. S. ANAEROBIC FORMATE OXIDATION: A FERREDOXIN-DEPENDENT REACTION. Science. 1964 Apr 17;144(3616):297–298. doi: 10.1126/science.144.3616.297. [DOI] [PubMed] [Google Scholar]
- BUCHANAN B. B., LOVENBERG W., RABINOWITZ J. C. A comparison of clostridial ferredoxins. Proc Natl Acad Sci U S A. 1963 Mar 15;49:345–353. doi: 10.1073/pnas.49.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARNAHAN J. E., CASTLE J. E. Some requirements of biological nitrogen fixation. J Bacteriol. 1958 Feb;75(2):121–124. doi: 10.1128/jb.75.2.121-124.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan M. P., Rajagopalan K. V., Handler P. The role of molybdenum in xanthine oxidase and related enzymes. Reactivity with cyanide, arsenite, and methanol. J Biol Chem. 1969 May 25;244(10):2658–2663. [PubMed] [Google Scholar]
- DAVISON D. C. Studies on plant formic dehydrogenase. Biochem J. 1951 Sep;49(4):520–526. doi: 10.1042/bj0490520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama T., Ordal E. J. Induced Biosynthesis of Formic Hydrogenlyase in Iron-Deficient Cells of Escherichia coli. J Bacteriol. 1965 Sep;90(3):673–680. doi: 10.1128/jb.90.3.673-680.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. Formic dehydrogenase of Bacterium coli: its inactivation by oxygen and its protection in the bacterial cell. Biochem J. 1939 Jun;33(6):1012–1027. doi: 10.1042/bj0331012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHSTEIN H. C., BOYD R. B. The formic hydrogenlyase system of Aerobacter aerogenes. J Bacteriol. 1953 May;65(5):617–618. doi: 10.1128/jb.65.5.617-618.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINNANE A. W., WRIGLEY C. W. FRAGMENTATION OF THE ELECTRON TRANSPORT CHAIN OF ESCHERICHIA COLI. PREPARATION OF A SOLUBLE FORMATE DEHYDROGENASE-CYTOCHROME B1 COMPLEX. Biochim Biophys Acta. 1963 Nov 8;77:408–418. doi: 10.1016/0006-3002(63)90515-8. [DOI] [PubMed] [Google Scholar]
- Li L. F., Ljungdahl L., Wood H. G. Properties of Nicotinamide Adenine Dinucleotide Phosphate-Dependent Formate Dehydrogenase from Clostridium thermoaceticum. J Bacteriol. 1966 Aug;92(2):405–412. doi: 10.1128/jb.92.2.405-412.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHEWS M. B., VENNESLAND B. Enzymic oxidation of formic acid. J Biol Chem. 1950 Oct;186(2):667–682. [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- Mazelis M. Formate Oxidation by Particulate Preparations from Higher Plants. Plant Physiol. 1960 May;35(3):386–391. doi: 10.1104/pp.35.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINSENT J. The need for selenite and molybdate in the formation of formic dehydrogenase by members of the coli-aerogenes group of bacteria. Biochem J. 1954 May;57(1):10–16. doi: 10.1042/bj0570010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri S. M., Hoare D. S. Formic hydrogenlyase and the photoassimilation of formate by a strain of Rhodopseudomonas palustris. J Bacteriol. 1968 Jun;95(6):2344–2357. doi: 10.1128/jb.95.6.2344-2357.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAEBURN S., RABINOWITZ J. C. PYRUVATE SYNTHESIS BY A PARTIALLY PURIFIED ENZYME FROM CLOSTRIDIUM ACIDI-URICI. Biochem Biophys Res Commun. 1965 Feb 3;18:303–307. doi: 10.1016/0006-291x(65)90703-5. [DOI] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- Sobel B. E., Lovenberg W. Characteristics of Clostridium pasteurianum ferredoxin in oxidation-reduction reactions. Biochemistry. 1966 Jan;5(1):6–13. doi: 10.1021/bi00865a002. [DOI] [PubMed] [Google Scholar]
- Sun A. Y., Ljungdahl L., Wood H. G. Total synthesis of acetate from CO2. II. Purification and properties of formyltetrahydrofolate synthetase from Clostridium thermoaceticum. J Bacteriol. 1969 May;98(2):842–844. doi: 10.1128/jb.98.2.842-844.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C. BACTERIAL FERREDOXIN. Bacteriol Rev. 1964 Dec;28:497–517. doi: 10.1128/br.28.4.497-517.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C., BRILL W. J., SAGERS R. D. FERREDOXIN LINKED DPN REDUCTION BY PYRUVATE IN EXTRACTS OF CLOSTRIDIUM ACIDI-URICI. Biochem Biophys Res Commun. 1963 Aug 1;12:315–319. doi: 10.1016/0006-291x(63)90303-6. [DOI] [PubMed] [Google Scholar]


