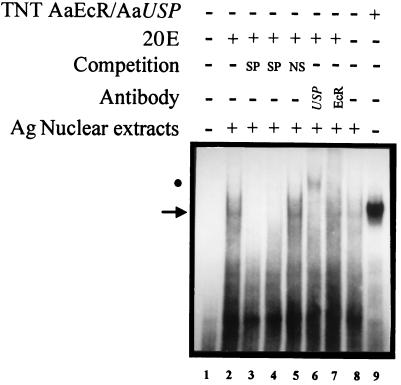
Figure 5.
Nuclear protein extracts from A. gambiae contain the ecdysone receptor complex EcR/USP. Nuclear extracts from whole adults of A. gambiae were examined by EMSA with AgEcRE-60 as a probe. The retarded complex resulting from the specific interaction between AgEcR/AgUSP and AgEcRE-60 is indicated by an arrow (lane 2). One hundred-fold molar excess of unlabeled probe (lane 3) or hsp27 EcRE (lane 4) was included as a specific competitor (SP). The same molar excess of unlabeled double-stranded oligonucleotide of unrelated sequence (NS) was included in lane 5. The supershift assay was performed with anti-USP (lane 6) and anti-EcR (lane 7). The effect of the absence of 20E in the binding reaction is presented in lane 8. As a control, a binding reaction using in vitro-translated AaEcR/AaUSP was included (lane 9). The positions of the supershifted complex (USP) is indicated by a solid circle. It should be noted that the antibodies are derived from species different than A. gambiae (26); anti-USP is derived from D. melanogaster and the anti-EcR is derived from A. aegypti, which could explain the partial supershift of the A. gambiae double-stranded oligonucleotide.