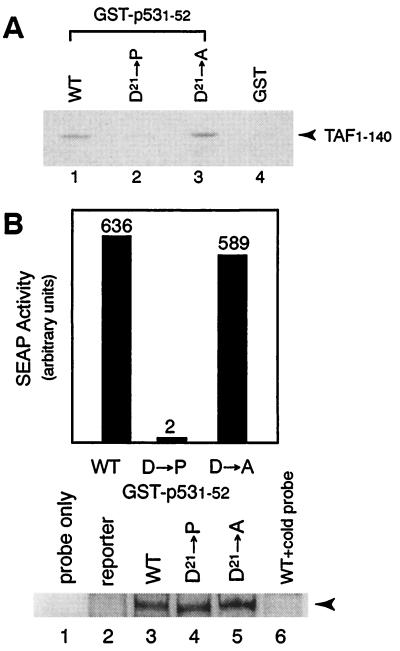
Figure 4.
Helix disruption greatly impairs TAF binding and transcriptional activation. (A) In vitro protein–protein interaction assays. Asp21 at the third position of the FXXΦΦ motif in the p53 activation domain was substituted with helix-breaking Pro. This substitution greatly reduced the binding affinity for TAF1–140 (compare lanes 1 and 2), whereas substitution of Asp21 with Ala had no significant effect on TAF binding (lane 3). TAF1–140 is not retained on GST resin (lane 4). The position of TAF1–140 is indicated by an arrowhead. (B) Transient transfection assays. Expression plasmids encoding GAL4-p531–52 and its mutants (D21 → P and D21 → A) were transiently transfected into Jurkat cells together with a reporter plasmid in which the production of secreted alkaline phosphatase (SEAP) is under control of an IL-2 promoter carrying five GAL4-binding sites. Gel mobility-shift analyses of the cell lysates also were performed to assess the expression levels of the mutant proteins. Lane 2 shows a control with lysate of the cells transfected only with the reporter plasmid. In lane 6, an excess of unlabeled probe was used as competitor. The position of the protein–DNA complex is indicated by an arrowhead.