Abstract
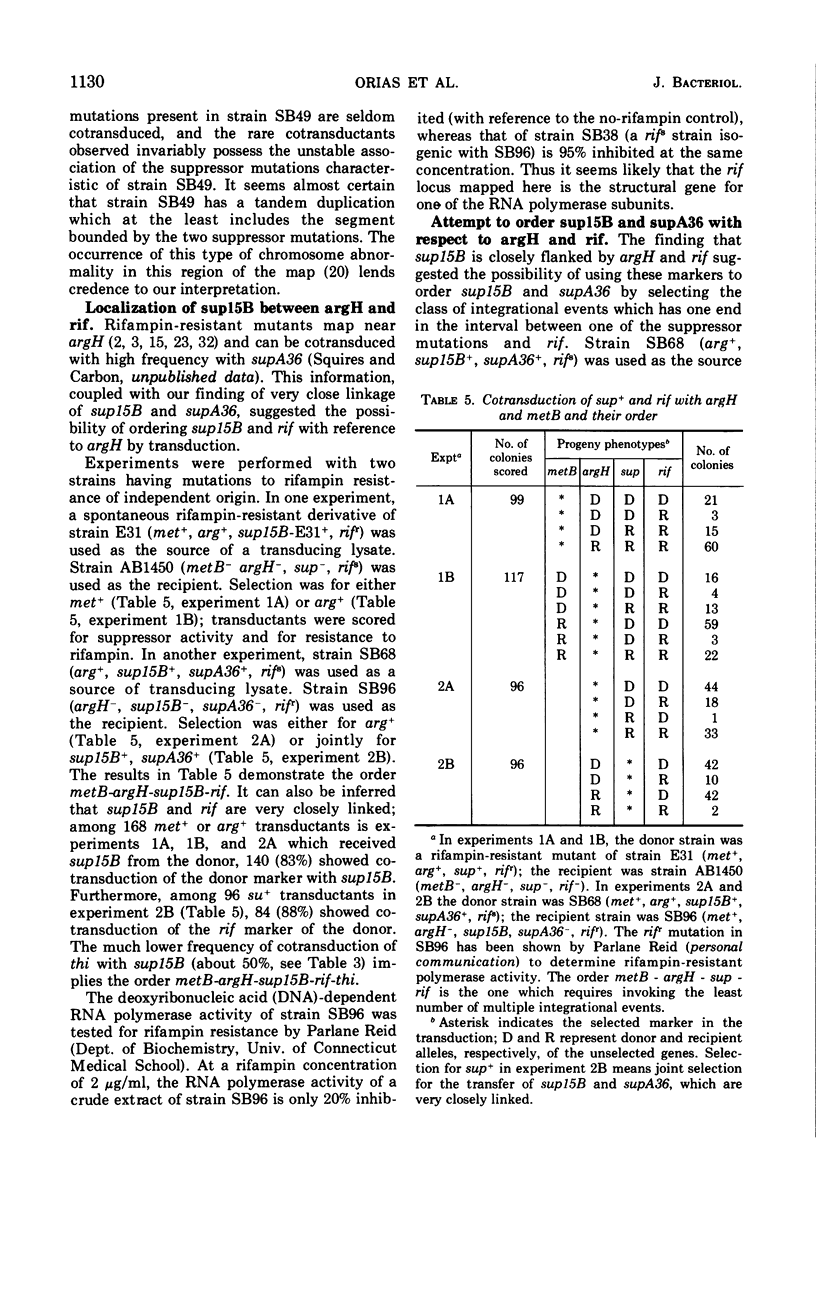
It was previously shown that the ochre suppressor mutation sup15B in Escherichia coli determines the accumulation of altered 30S ribosomal subunits and the presence of altered transfer ribonucleic acid (tRNA) capable of suppressing in vitro the UAG codon. This mutation has been mapped in the present study by means of conjugation and transduction experiments. After establishing the location of sup15B near argH, the following order was determined for the markers tested: metB-argH-(sup15B, supA36)-rif-thi. A comparison of location, growth rate, and suppressor pattern determined by sup15B and supM indicates the high probability that both suppressor mutations are identical. This study has also yielded a more precise location for the rifampin resistance gene. The most interesting finding is the very close (if not adjacent) location of the suppressor mutations sup15B and supA36, both of which determine tRNA alterations.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S. Isolation of tyrosine tRNA precursor molecules. Nat New Biol. 1971 Jan 6;229(1):19–21. doi: 10.1038/newbio229019a0. [DOI] [PubMed] [Google Scholar]
- Austin S., Scaife J. A new method for selecting RNA polymerase mutants. J Mol Biol. 1970 Apr 14;49(1):263–267. doi: 10.1016/0022-2836(70)90394-3. [DOI] [PubMed] [Google Scholar]
- BRODY S., YANOFSKY C. Suppressor gene alteration of protein primary structure. Proc Natl Acad Sci U S A. 1963 Jul;50:9–16. doi: 10.1073/pnas.50.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt D., Darnell J. E., Jr tRNA synthesis in HeLa cells: a precursor to tRNA and the effects of methionine starvation on tRNA synthesis. J Mol Biol. 1969 May 28;42(1):43–56. doi: 10.1016/0022-2836(69)90485-9. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Carbon J., Squires C., Hill C. W. Genetically altered tRNA-Gly subspecies in E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:505–512. doi: 10.1101/sqb.1969.034.01.057. [DOI] [PubMed] [Google Scholar]
- Carbon J., Squires C., Hill C. W. Glycine transfer RNA of Escherichia coli. II. Impaired GGA-recognition in strains containing a genetically altered transfer RNA; reversal by a secondary suppressor mutation. J Mol Biol. 1970 Sep 28;52(3):571–584. doi: 10.1016/0022-2836(70)90420-1. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza L L, Lederberg J. Isolation of Pre-Adaptive Mutants in Bacteria by Sib Selection. Genetics. 1956 May;41(3):367–381. doi: 10.1093/genetics/41.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel V., Sarid S., Beckmann J. S., Littauer U. Z. In vitro transcription of a transfer RNA gene. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1260–1266. doi: 10.1073/pnas.66.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsson G., Adelberg E. A. Map positions and specificities of suppressor mutations in Escherichia coli K-12. Genetics. 1965 Aug;52(2):319–340. doi: 10.1093/genetics/52.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsson G. Mapping of ochre suppressors in Escherichia coli. Genet Res. 1968 Feb;11(1):15–20. doi: 10.1017/s0016672300011150. [DOI] [PubMed] [Google Scholar]
- Ezekiel D. H., Hutchins J. E. Mutations affecting RNA polymerase associated with rifampicin resistance in Escherichia coli. Nature. 1968 Oct 19;220(5164):276–277. doi: 10.1038/220276a0. [DOI] [PubMed] [Google Scholar]
- GARTNER T. K., ORIAS E. PLEIOTROPIC EFFECTS OF SUPPRESSORS OF A LAC-"OPERATOR NEGATIVE" MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1965 Jan;53:62–68. doi: 10.1073/pnas.53.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner T. K., Orias E. Effects of mutations to streptomycin resistance on the rate of translation of mutant genetic information. J Bacteriol. 1966 Mar;91(3):1021–1028. doi: 10.1128/jb.91.3.1021-1028.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner T. K., Orias E., Lannan J. E., Beeson J., Reid P. J. The molecular basis of suppression in an ochre suppressor strain possessing altered ribosomes. Proc Natl Acad Sci U S A. 1969 Mar;62(3):946–951. doi: 10.1073/pnas.62.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYES W. The kinetics of the mating process in Escherichia coli. J Gen Microbiol. 1957 Feb;16(1):97–119. doi: 10.1099/00221287-16-1-97. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Khesin R. B., Gorlenko Z. M., Shemyakin M. F., Stvolinsky S. L., Mindlin S. Z., Ilyina T. S. Studies on the functions of the RNA polymerase components by means of mutations. Mol Gen Genet. 1969;105(3):243–261. doi: 10.1007/BF00337475. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- PITTARD J., RAMAKRISHNAN T. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI. IV. EFFECT OF A CHROMOSOMAL DELETION ON CHROMOSOME TRANSFER. J Bacteriol. 1964 Aug;88:367–373. doi: 10.1128/jb.88.2.367-373.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P., Berg P. T4 bacteriophage mutants suppressible by a missense suppressor which inserts glycine in place of arginine for the codon AGA. J Virol. 1968 Sep;2(9):905–914. doi: 10.1128/jvi.2.9.905-914.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P., Gartner T. K., Orias E. Abnormal 30S ribosomal subunits determined by an ochre suppressor mutation in Escherichia coli. J Bacteriol. 1969 Apr;98(1):308–310. doi: 10.1128/jb.98.1.308-310.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Squires C., Carbon J., Hill C. W. Glycine transfer RNA of Escherichia coli. I. Structural genes for two glycine tRNA species. J Mol Biol. 1970 Sep 28;52(3):557–569. doi: 10.1016/0022-2836(70)90419-5. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Marino P., Colvill A. J. Mutant of E. coli containing an altered DNA-dependent RNA polymerase. Nature. 1968 Oct 19;220(5164):275–276. doi: 10.1038/220275a0. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Engelhardt D. L., Zinder N. D., Konigsberg W. Amber mutants and chain termination in vitro. J Mol Biol. 1967 Oct 14;29(1):27–43. doi: 10.1016/0022-2836(67)90179-9. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J., Horn V. Amino acid replacements and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:151–162. doi: 10.1101/sqb.1966.031.01.023. [DOI] [PubMed] [Google Scholar]
- Yura T., Igarashi K. RNA polymerase mutants of Escherichia coli. I. Mutants resistant to streptovaricin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1313–1319. doi: 10.1073/pnas.61.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]



