Fig. (1).
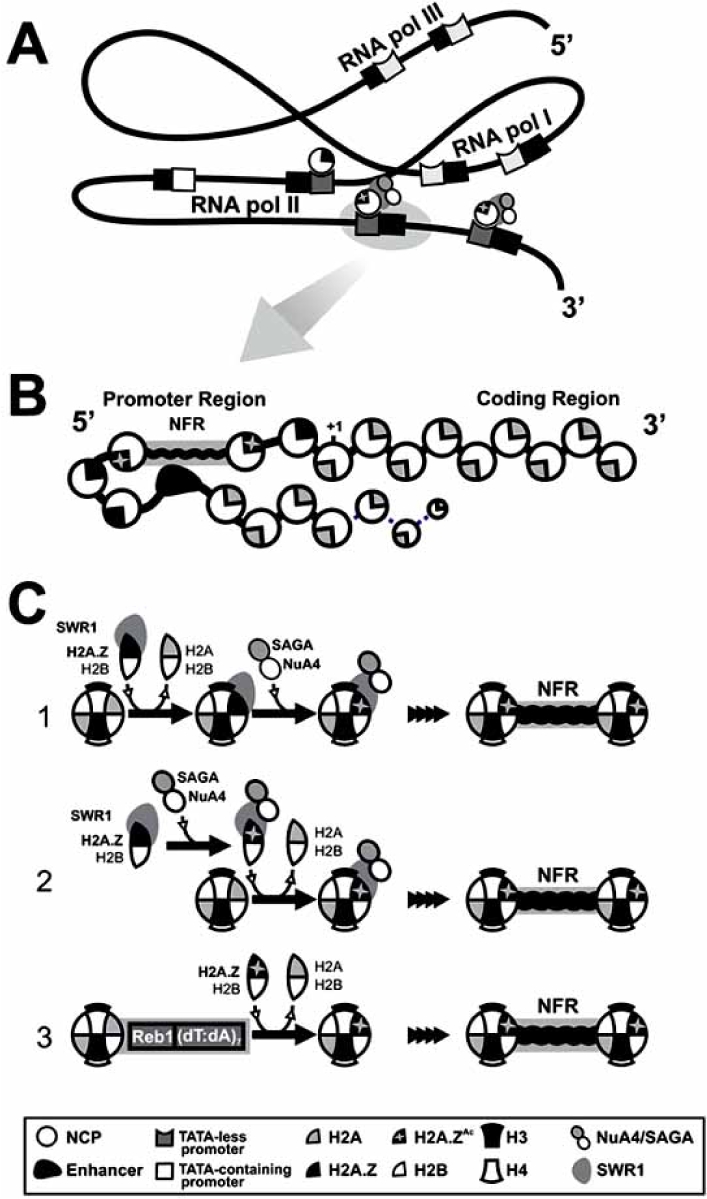
Schematic representation of the H2A.Z genome-wide distribution and the different mechanisms of chromatin recruitment. A) H2A.Z is preferentially enriched at TATA-less promoters of genes transcribed by RNA polymerase II [17∗∗]. Targeting of H2A.Z to these regions is reliant on SWR1, and involves acetylation of N-terminal lysines by NuA4 and SAGA (mostly K14 in the case of yeast [15∗]). B) Nearly all promoters of yeast genes contain an ~ 150 bp nucleosome-free region (NFR) centered about ~ 200 bp upstream of the initiation codon, which contains the transcription initiation site flanked by two H2A.Z-containing nucleosomes. Although H2A.Z occupation at promoters is not correlated with transcriptional activity, H2A.Z-K14Ac is preferentially enriched at promoters of active genes [22∗∗], raising the possibility that acetylated H2A.Z may contribute to nucleosome loss during activation, together with acetylation of other members of the core histone complement. C) H2A.Z is acetylated in vivo by NuA4 and SAGA [22∗∗, 27∗∗-29]. Different studies have reached different conclusions as it pertains to the role of acetylation in H2A.Z deposition in chromatin. One possible mechanism (C.1) involves the acetylation of H2A.Z only after (Legend Fig. 1) contd…. it is recruited into chromatin by SWR1 [28∗]. By contrast, a second model (C.2) has been proposed in which H2A.Z deposition would require its previous acetylation and thus, acetylation itself would regulate H2A.Z recruitment [22∗∗]. In addition, a recent report [16∗∗] has demonstrated that H2A.Z can be recruited to specific chromatin regions regardless of SWR1 and acetylation, through the recognition of specific DNA elements (the Reb1 signal and a dT:dA tract) at promoters of both active and inactive genes (C.3). The recognition of these elements induces the formation of the NFR region flanked by two positioned H2A.Z-containing nucleosomes and it thus, complements the recruitment pathways reliant on acetylation and SWR1 (C.1, C.2).
