Fig. 5.
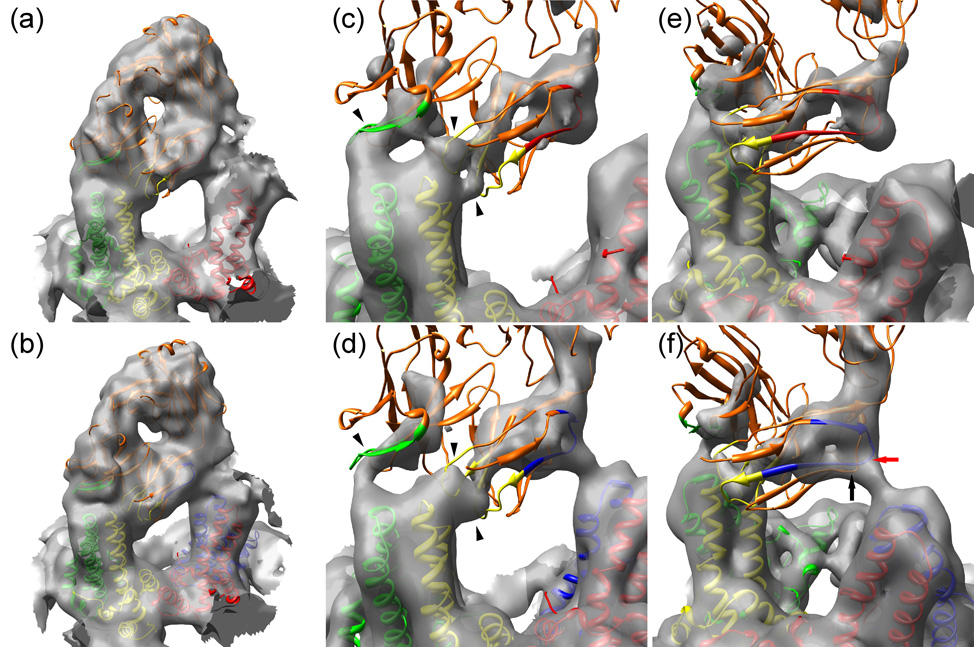
Models of the interactions of Fab 9c8 with HBV capsids. (Panels (a) and (b)) The composite atomic model of Fab 9c8 is shown docked into the electron density at the AB dimer of T=3 (a) and T=4 (b) capsids. The capsid subunits are color-coded as in Fig. 2. The Fab is colored orange with the interacting CDRs highlighted in the same color as the capsid monomer with which they make contact: (heavy chain CDR2, green), and light chain CDRs 1 and 3 (yellow). (Panels (c) and (d)) Closer views of the Fab CDRs engaging the spike tip, and contoured at a high level. The Fab contacts the T=3 AB dimer at three sites (arrowheads) whereas these three points are not as well resolved on T=4 capsids. (Panels (e) and (f)) As in panels (c) and (d) but viewed with the interaction between the Fab framework and the adjacent spike in the foreground on the right. A portion of the FR1 sequences is highlighted, colored the same as the capsid monomer with which they make contact. T=3 capsids do not have a D quasi-equivalent site, and consequently the Fab contacts a C monomer (red) (located in the background but not shown here for clarity), whereas a Fab on a T=4 capsid contacts a D monomer (blue). The points on the FR1 and FR3 sequences making the closest approach to the D monomer are indicated by red and black arrows, respectively.
