Fig. 6.
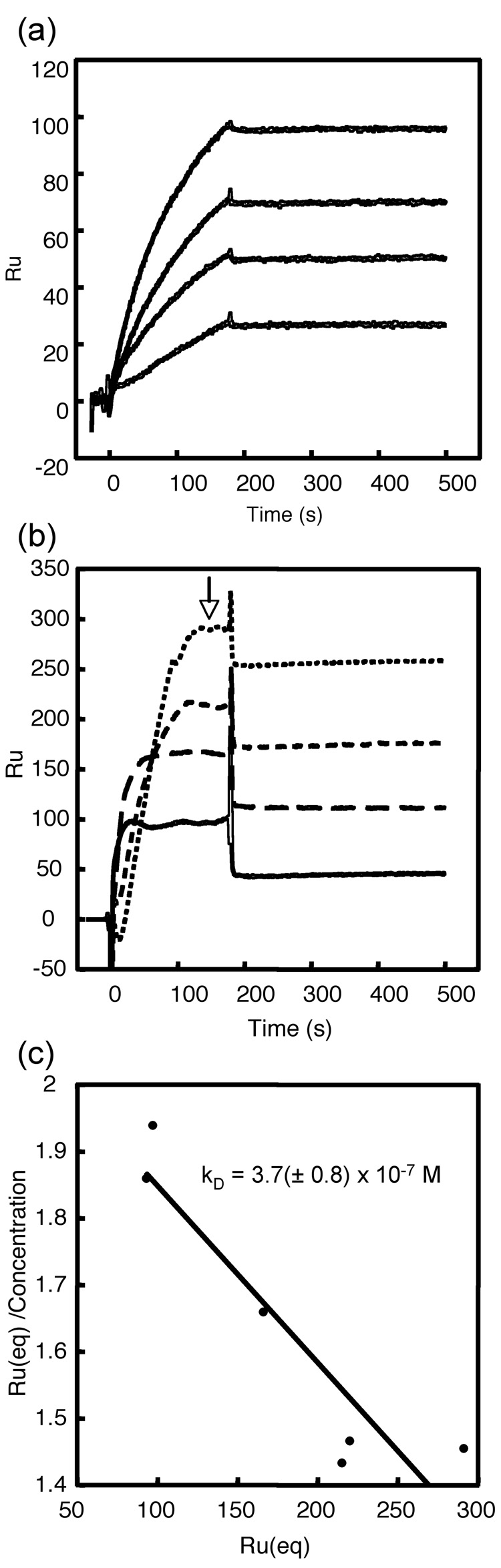
Measurement of the kinetics and affinity of Fabs binding to HBcAg by surface plasmon resonance. (a) Sensograms of F11A4 binding to capsids (bottom to top: 25, 50, 75, and 100 nM analyte). Binding is characterized by an intermediate on-rate but a very low off-rate, accounting for the high affinity of this antibody (KD ~ 1 × 10−10 (M)). (b) Sensograms of Fab 9c8 binding to capsids (bottom to top: 50, 100, 150, and 200 nM analyte). Binding is characterized by an intermediate on-rate but a high off-rate, resulting in a relatively low affinity. Replicates not shown for clarity. Because the high off-rates of 9c8 prevented a conventional simultaneous analysis of the sensograms, the degree of binding at equilibrium (open arrow) as a function of concentration was monitored instead. (c) A Scatchard plot of the equilibrium binding gives a KD of ~ 4 × 10−7 (M).
