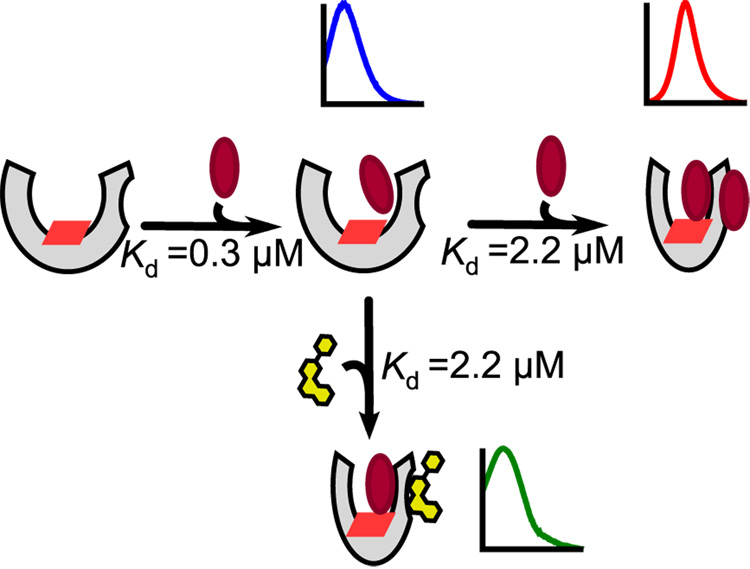
Figure 7.
Proposed model for Nile Red allosterism. Nile Red (maroon ellipse) binds with high affinity to the active site. Lower-affinity binding of a second Nile Red molecule at a peripheral effector binding site induces a conformational change that increases the accessibility of the active-site Nile Red molecule to the heme-iron center (red parallelogram). In contrast, effector molecules such as ANF (yellow) bind with higher affinity to the peripheral site, and can form a mixed ternary complex with CYP3A4 and Nile Red; the photophysical properties of Nile Red may provide insight into the nature of this mixed complex for different effectors.