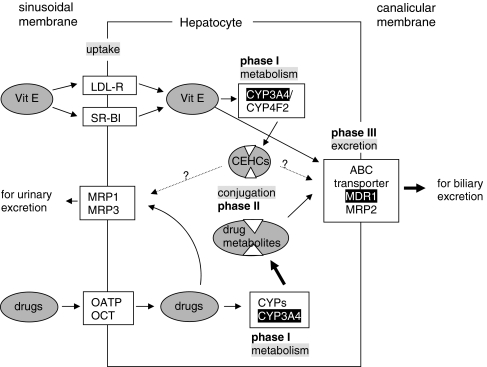
Fig. 2.
Metabolism of vitamin E follows the metabolism of xenobiotics. Drugs are mainly taken up in the liver by the organic anion transporting peptide (OATP) or the organic cation transporters (OCT) at the sinusoidal membrane. All forms of chylomicron-remnant-bound vitamin E enter the hepatocyte via the LDL-receptor, HDL-bound α-tocopherol via the scavenger receptor BI (SR-BI). Within the cell the mainly lipophilic drugs become “activated” by phase I enzymes which oxidize, reduce or hydrolyze drugs before they are subjected to phase II metabolism. Oxidation/hydroxylation is catalyzed by cytochrome P450 enzymes, mainly by CYP3A4 in humans, Cyp3a11 in mice and CYP3A1 in rats. Tocopherols and tocotrienols are hydroxylated in human cells by CYP3A4 or CYP4F2. The hydroxy group is oxidized by ω-oxidation to the carboxy group, then the side chain is degraded by β-oxidation ending up in carboxyethyl hydroxychromans (CEHC), see Fig. 1. Metabolites of drugs and vitamin E are sulphated or glucuronidated during phase II metabolism to make them more hydrophilic for the excretion via the biliary or the renal route. Efflux into the bile occurs via the ABC transporters MDR1 or MRP2. Intact vitamin E appears to take this route, too. MRP1 and MRP3 are located at the sinusoidal membrane and pump drugs and their metabolites into the circulation for renal excretion. Whether also the CEHCs are transported via ABC transporters remains to be investigated. Enzymes/transporters shown to be up-regulated by α-tocopherol are indicated white on black background, stimulated pathways by bold arrows. It is obvious that induction of CYP3A4 expression increases the phase I metabolism of drugs. Moreover, induction of MDR-1 is followed by an enhanced excretion of drug metabolites. In consequence, the efficacy of a given dosage of the respective drug will decrease