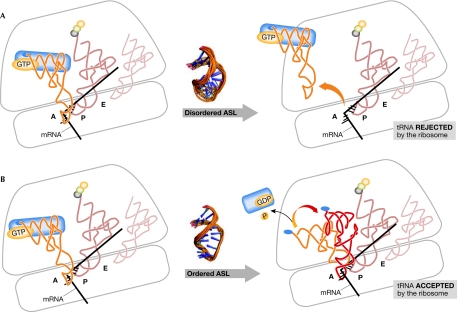
Figure 3.
Anticodon recognition and transfer RNA accommodation on the ribosome. (A) The complex of aminoacylated transfer RNA (tRNA) bound to elongation factor/GTP enters the A-site (left). The anticodon of the tRNA makes contact with the messenger RNA (mRNA) codon; however, the tRNA with a disordered anticodon stem and loop domain (ASL) cannot contact the codon correctly and is rejected by the ribosome (right). An aminoacyl-tRNA having successfully received the growing peptide now occupies the P-site, while the tRNA from which it received the nascent protein occupies the ribosome's exit or E-site. (B) Accommodation of aminoacyl-tRNA in the A-site. A complex of aminoacylated tRNA with elongation factor and GTP enters the A-site (left). Modifications have restricted the dynamics and shaped the architecture of the ASL of the tRNA. With recognition that the tRNA has the correct anticodon, the 16S ribosomal RNA (rRNA) nucleosides A1492, A1493 and G530 hydrogen bond to the backbones of the mRNA and the anticodon. The small ribosomal subunit and the tRNA undergo conformational changes. The conformation of the tRNA above the ASL changes, but the architecture of the ASL and its interaction with the codon remain unchanged (right). GTP is hydrolysed and the elongation factor-GDP leaves the ribosome.