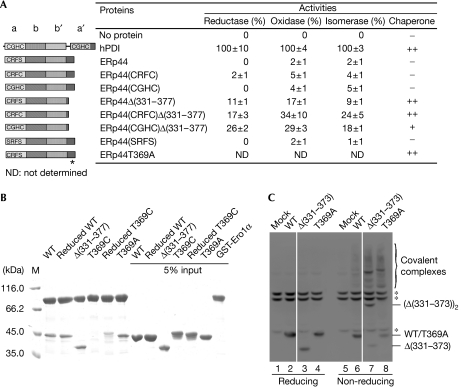
Figure 4.
The C-terminal tail of human ERp44 restricts substrate binding in vitro and in vivo. (A) Schematic representation of the wild-type and mutant ERp44 proteins with the CRFS motif mutated and/or with the C-terminal (residues 331–377) truncated or mutated is shown on the left, and their enzyme and chaperone activities on the right. Data of enzyme activities are expressed as mean±s.d. (n=3), and inhibition of rhodanese aggregation as no activity (–), weak activity (+) and strong activity (++). The asterisk indicates the T369A mutation site. (B) Pull-down assay of ERp44 proteins. Glutathiol Sepharose 4 fast-flow beads (GE Healthcare, Buckinghamshire, UK) were incubated at 25°C and pH 7.5 for 1 h with 10−6 M GST-Ero1α and various ERp44 proteins, as indicated, and then analysed by SDS–12% polyacrylamide gel electrophoresis (SDS–12% PAGE) under reducing conditions after being washed five times. (C) The tail deletion (ERp44Δ(331–373)) and replacement (ERp44T369A) mutants accumulate intracellularly mostly as mixed disulphides with client proteins. Lysates from HeLa cells transiently transfected with the indicated vectors were prepared 48 h after transfection and resolved by SDS–12% PAGE under reducing (lanes 1–4) or non-reducing (lanes 5–8) conditions, blotted and decorated with monoclonal HA antibodies. The asterisks indicate anti-HA reactive background bands, present also in mock-transfected cells. White lines separate lanes 2 and 3, and 6 and 7, which were juxtaposed from the same gel. ERp44, human ERp44; HA, haemagglutinin epitope tag; M, marker; WT, wild-type ERp44; T369C, ERp44T369C; T369A, ERp44T369A; Δ(331–373), ERp44Δ(331–373); Δ(331–377), ERp44Δ(331–377).