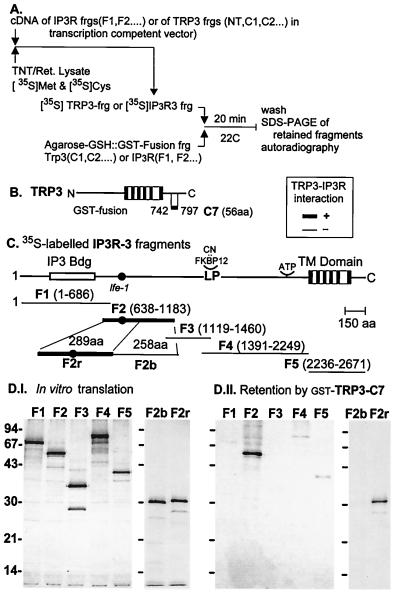
Figure 2.
Binding of IP3 receptor fragments to human TRP3. In vitro-translated IP3R3 F1–F5 fragments, labeled with [35S]Met and [35S]Cys, were incubated with a TRP segment fused to GST and adsorbed to glutathione-Sepharose. After 30 min at room temperature, Sepharose beads were washed, and the adsorbed complexes were resolved by SDS/PAGE, stained, and autoradiographed. In other experiments, the inverse strategy was used: the in vitro-translated fragment was from hTRP3, and the protein fused to GST was from the human IP3R3. (A) Design of the GST-pulldown experiments. (B) Linear diagram of the IP3R3 and TRP3, represented with the same scale and location of some of the molecular landmarks of IP3R3. (C) Representative results showing binding of F2, but not the other IP3R fragments, to TRP-C7.