Abstract
Germline stem cells, which can self-renew and generate gametes, are unique stem cells in that they are solely dedicated to transmit genetic information from generation to generation. The germ cells have a special place in the life cycle because they must be able to retain the ability to recreate the organism, a property known as developmental totipotency. Several lines of evidence have suggested the extensive proliferation activity and pluripotency of prenatal, neonatal and adult germline stem cells. We showed that adult male germline stem cells, spermatogonial stem cells, can be converted into embryonic stem cell-like cells, which can differentiate into the somatic stem cells of three germ layers. Different cell types such as vascular, heart, liver, pancreatic and blood cells could also be obtained from these stem cells. Understanding how spermatogonial stem cells can give rise to pluripotent stem cells and how somatic stem cells differentiate into germ cells could give significant insight into the regulation of developmental totipotency as well as having important implications for male fertility and regenerative medicine.
Keywords: germline stem cells, pluripotency, regenerative medicine, spermatogonial stem cells, totipotency
Introduction: development of germ cells
The formation of germ cells during embryogenesis is of ultimate importance for the continuation of every species. Development of germ cells begins with the specification of the founder cells of germ cells, primordial germ cells. Primordial germ cell (PGC) specification or formation marks the initiation of the life cycle of the germ cell lineage in all species. In mammals, signals from extraembryonic tissues are critical for the specification of PGCs. Precursors of PGCs were localized in the proximal region of the epiblast and all clones which gave rise to PGCs also differentiated to the extraembryonic mesoderm (Lawson & Hage, 1994).
In the mouse, PGCs are first distinguishable at embryonic day 7.25–7.5 within the extraembryonic mesoderm in the distal portion of the primitive streak and at the base of allantoic buds. They are normally identified by virtue of high membrane alkaline phosphatase (ALP) activity (Ginsburg et al. 1990).
Following allocation, PGCs start to migrate from the base of the allantois toward the genital ridges. At the same time, PGCs undergo active mitosis, during which, as has been well documented in mice, a number of environmental factors affect their growth and survival (Anderson et al. 1991). For the period of their proliferation, PGCs may autonomously and instructively differentiate and change their character. Although no obvious sexual differences have been observed during the initial specification and proliferation phases, male and female germ cells assume distinct paths of differentiation during embryogenesis, and even more so during spermatogenesis and oogenesis.
The development of post-migratory germ cells, including postnatal periods, is sex-specific. Germ cells in the female enter meiosis and undergo meiotic arrest until after birth. Germ cells in the male enter mitotic arrest and are reactivated to initiate spermatogenesis after birth (Wylie, 1999). Spermatogenesis continues during adult life in most males, but whether these stem cells self-renew or differentiate is heavily influenced by surrounding somatic cells, in a microenvironment often referred to as a stem cell niche. After birth, male germ cells differentiate to spermatogonial stem cells. The continuation of the spermatogenic process throughout life relies on the proper regulation of self-renewal and differentiation of the spermatogonial stem cells. These are single cells situated on the basal membrane of the seminiferous epithelium. Only 0.03% of all germ cells are spermatogonial stem cells (Nayernia et al. 2004; Olive & Cuzin, 2005; Kubota & Brinster, 2006).
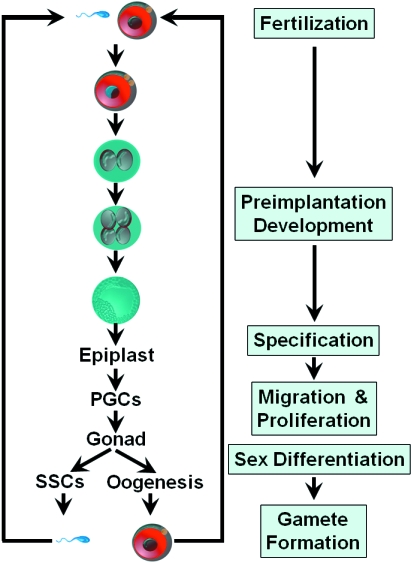
The life cycle of germ cells is shown in Fig. 1. There are three critical events during the life cycle of germ cells: specification, migration/proliferation, and pre- and postnatal sex-specific development (Fig. 1).
Fig. 1.
The cycle of germ cell development. Following germ line specification, PGCs appear first in the extraembryonic mesoderm. PGCs migrate to the genital ridge and actively proliferate. The development of postmigratory germ cells is sex-specific. Female germ cells enter meiosis and undergo meiotic arrest until after birth. Male germ cells enter mitotic arrest and are reactivated to initiate spermatogenesis after birth. Male germ cells develop to spermatogonial stem cells (SSCs), which differentiate to sperm after a highly complicated differentiation process.
Potency of germ cells
In an early embryo a cell has the potential to generate many different cell types. During development cells generally lose this potential or ‘potency’, and become restricted to making one or a few cell types. Cells can become undetermined and undifferentiated under special circumstances. These undifferentiated cells, which are capable of self-renewal and differentiate into more specialized cells, are termed stem cells (Reik et al. 2001; Gardner, 2002). Stem cells have the remarkable potential to develop into many different cell types in the body. Serving as a sort of repair system for the body, they can theoretically divide without limit to replenish other cells as long as the person or animal is still alive. When a stem cell divides, each new cell has the potential either to remain a stem cell or to become another type of cell with a more specialized function, such as a muscle cell, a red blood cell or a brain cell. Embryonic stem cells, as their name suggests, are derived from embryos.
There are several lines of evidence which show the potency of germ cells (Donovan, 1998; Donovan & de Miguel, 2004; Kanatsu-Shinohara & Shinohara, 2006; Ratajczak et al. 2007).
Cultured PGCs exposed to a specific cocktail of growth factors give rise to embryonic germ cells, pluripotent stem cells that can contribute to all the lineages of chimeric embryos including the germline. The conversion of PGCs into pluripotent stem cells is a remarkably similar process to nuclear reprogramming in which a somatic nucleus is reprogrammed in the egg cytoplasm. The conversion of PGCs to pluripotent stem cells may be linked in some way to their deregulated proliferation (Cooke et al. 1993; McLean, 2006).
Other evidence is the pluripotency of testicular germ cell tumours. Testicular teratomas are highly unusual benign tumours containing derivatives of the three primary germ layers. Different studies have shown that embryonal carcinomas (ECs) are derived from PGCs (Stevens, 1967). ECs are pluripotent cells; however, malignant development of these tumours may ultimately correlate with a decrease in pluripotency, because this would tend to increase the propensity of EC cells for self-renewal. Understanding the genetics of embryonal carcinoma cell formation and the growth factor signalling pathways controlling embryonic germ cell derivation could tell us much about the molecular controls on developmental potency in mammals (Damjanov, 1990; Hussain, 2005).
Furthermore, pluripotency of postnatal germline stem cells was clearly demonstrated (Kanatsu-Shinohara et al. 2004; Guan et al. 2006; Seandel et al. 2007). After birth, male germline stem cells develop into spermatogonial stem cells (SSCs). The SSCs are at the foundation of spermatogenesis. They can self-renew and generate a large number of differentiated germ cells. These cells could be reprogrammed to embryonic stem cell-like cells and can spontaneously differentiate into derivates of all three germ layers in vitro. When injected into an early blastocyst, reprogrammed SSCs contribute to the development of various tissues.
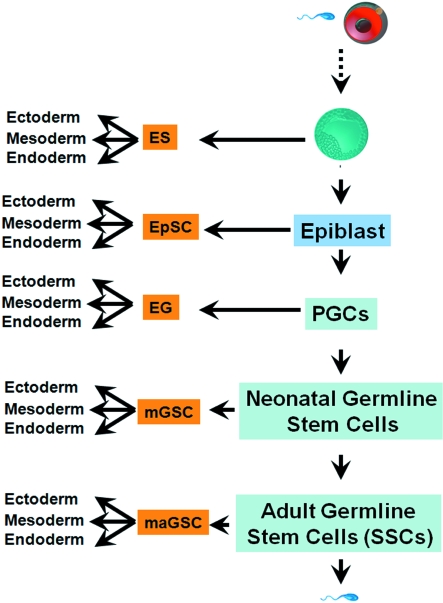
These lines of evidence clearly suggest the pluripotency of germ cells in all stages of development (Fig. 2).
Fig. 2.
The cycle of pluripotency. Germ cells are able to be reprogrammed to pluripotent stem cells in all stages of development. ES, embryonic stem cells; EpSC, epiblast stem cells; EG, embryonic germ cells; mGSCs, multipotent germline stem cells; maGSCs, multipotent germline stem cells.
Relevance of germ cell potency for regenerative medicine
Recent advances in cellular therapies have led to the emergence of a multidisciplinary scientific approach to developing therapeutics for a wide variety of diseases and genetic disorders. Although most cell-based therapies currently consist of heterogeneous cell populations, it is anticipated that the standard of care needs well-characterized stem cell lines that can be modified to meet the individual needs of the patient. Extensive research in the area of regenerative medicine is focused on the development of cells, tissues and organs for the purpose of restoring function through transplantation. The general belief is that replacement, repair and restoration of function is best accomplished by cells, tissues or organs that can provide the appropriate physiological/metabolic functions more efficiently than any mechanical devices, recombinant proteins or chemical compounds. Several cell-based strategies are currently being investigated, including cell preparations from autologous parenchyma or established cell lines, as well as cell therapies derived from a variety of stem cell sources such as bone marrow or cord blood stem cells, embryonic stem cells, as well as cells, tissues and organs from genetically modified animals. Several lines of evidence have suggested extensive proliferation activity and pluripotency of germline stem cells, including SSCs. These characteristics provide new and unprecedented opportunities for the therapeutic use of SSCs for regenerative medicine.
We have succeeded in developing a procedure for the isolation and purification of SSCs from adult mouse testis (Guan et al. 2006). We were able to isolate and culture these cells in culture medium containing the precise combination of cellular growth factors needed for the cells to reproduce themselves in vitro. These cells were characterized with regard to their molecular profiles and these were compared via molecular profiling of embryonic stem cells using a stem cell array which contains relevant genes related to stem cell metabolism. The results indicate that SSCs share many molecular characteristics with embryonic stem cells. On the cellular level, SSCs resemble embryonic stem cells; they form an embryoid body structure after 2 weeks of culture. Stem cell potential of isolated SSCs was examined using the transplantation technique. This method allowed SSCs to recolonize the seminiferous tubuli of germ cell-depleted mice and regenerate spermatogenesis. These cells are able to differentiate into various cell types of three germ layers in vitro (Guan et al. 2006; Guan et al. 2007). In contrast to ESCs, use of SSCs for cell transplantation will allow establishment of individual cell-based therapy, because the donor and recipient are identical. In addition, any ethical problems are avoided.
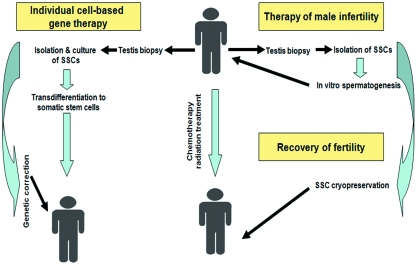
Our approach provides an accessible in vitro model system for studies of mammalian gametogenesis, as well as for developing new strategies for reproductive engineering, infertility treatment and establishment of individual cell-based therapy (Fig. 3).
Fig. 3.
Perspective of the cell therapeutic approaches using spermatogonial stem cells. Spermatogonial stem cells are pluripotent stem cells and can be differentiated to different cell types, including sperm. Human SSCs can be used in cell-based regenerative medicine. SSCs can be used for therapy of genetically determined diseases, if homologous recombination in the cells works successfully. Finally, SSCs can recover fertility of cancer patients after chemotherapy and radiation treatment.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG) and University of Newcastle upon Tyne. We thank Hamed Nayernia for the excellent informative graphics.
References
- Anderson O, Heasman J, Wylie C. Early events in the mammalian germ line. Int Rev Cytol. 2001;203:215–230. doi: 10.1016/s0074-7696(01)03008-x. [DOI] [PubMed] [Google Scholar]
- Cooke JE, Godin I, Ffrench-Constant C, et al. Culture and manipulation of primordial germ cells. Methods Enzymol. 1993;225:37–58. doi: 10.1016/0076-6879(93)25006-n. [DOI] [PubMed] [Google Scholar]
- Damjanov I. Teratocarcinoma stem cells. Cancer Surv. 1990;2:303–319. [PubMed] [Google Scholar]
- Donovan PJ. The germ cell – the mother of all stem cells. Int J Dev Biol. 1998;42:1043–1050. [PubMed] [Google Scholar]
- Donovan P, de Miguel M. Turning germ cells into stem cells. Curr Opin Genet Dev. 2004;13:463–471. doi: 10.1016/j.gde.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Stem cells: potency, plasticity and public perception. J Anat. 2002;3:277–282. doi: 10.1046/j.1469-7580.2002.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Guan K, Wagner S, Unsold B, et al. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–1625. doi: 10.1161/01.RES.0000269182.22798.d9. [DOI] [PubMed] [Google Scholar]
- Hussain A. Germ cell tumors. Curr Opin Oncol. 2005;17:268–274. doi: 10.1097/01.cco.0000160750.33552.42. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Shinohara T. The germ of pluripotency. Nat Biotechnol. 2006;24:663–664. doi: 10.1038/nbt0606-663. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1112. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kubota H, Brinster RL. Technology insight: in vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat Clin Pract Endocrinol Metab. 2006;2:99–108. doi: 10.1038/ncpendmet0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Hage WJ. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found Symp. 1994;182:68–84. doi: 10.1002/9780470514573.ch5. [DOI] [PubMed] [Google Scholar]
- McLean DJ. Vertebrate reproductive stem cells: recent insights and technological advances. Semin Cell Dev Biol. 2006;17:534–539. doi: 10.1016/j.semcdb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Nayernia K, Li M, Engel W. Spermatogonial stem cells. Methods Mol Biol. 2004;253:105–120. doi: 10.1385/1-59259-744-0:105. [DOI] [PubMed] [Google Scholar]
- Olive V, Cuzin F. The spermatogonial stem cell: from basic knowledge to transgenic technology. Int J Biochem Cell Biol. 2005;37:246–250. doi: 10.1016/j.biocel.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Machalinski B, Wojakowski W, et al. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Seandel M, James D, Shmelkov SV, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LC. Origin of testicular teratomas from primordial germ cells in mice. J Natl Cancer Inst. 1967;38:549–552. [PubMed] [Google Scholar]
- Wylie C. Germ cells. Cell. 1999;96:165–174. doi: 10.1016/s0092-8674(00)80557-7. [DOI] [PubMed] [Google Scholar]