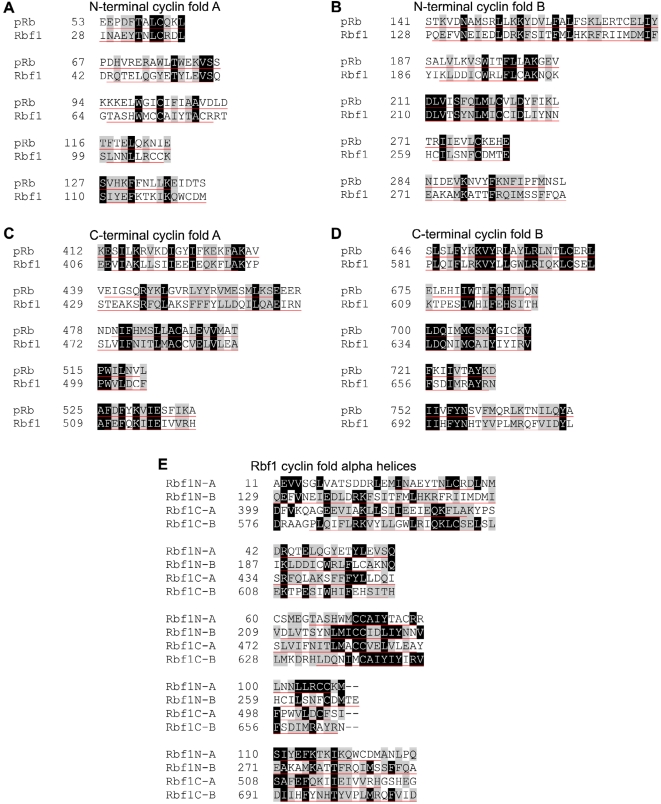
Figure 6. Alignment of Cyclin fold helices within the Rbf1 sequence.
The retinoblastoma proteins in humans and flies share a domain structure containing four cyclin folds, with each fold consisting of five alpha helices. The N-terminal (A and B) and C-terminal (C and D) domains of Rbf1 each have a cyclin fold A and B, resulting in four total cyclin folds that share extensive sequence conservation with pRb. It is likely that the retinoblastoma family of proteins emerged from two successive tandem duplication events from an ancient cyclin-like ancestor that gave rise to many cell cycle regulators. This finding seems to indicate that the retinoblastoma N and C-terminal domains are intrahomologues. The tandem domain architecture of Rb family proteins may explain our finding that ORC interacts with multiple Rbf1 domains, and suggests that Rbf1 may be an adaptor molecule that is able to switch between several orientations with ORC to accommodate different combinations of binding partners depending on different cellular contexts. (E) All five helices from the four Rbf1 cyclin folds were compared together. Amino acids conserved in two or more helices were shaded accordingly, revealing a collective conservation of amino acid sequence between the cyclin folds. Black shading with white letters indicates identical amino acids. Grey shading indicates amino acid similarity. Helices are underlined in red.