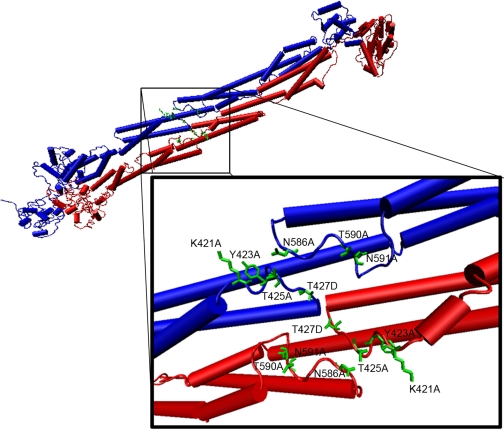
FIGURE 4.
Model representation of the smooth muscle α-actinin central rod spectrin repeat-like R2 and R3 domains and key loop residues. A structural model of the smooth muscle α-actinin R2 and R3 domains in the central rod (Liu et al. (32)) was modified to show positions of the R2 and R3 loop residues mutated for sm-titin Zq binding interaction experiments. One monomer peptide of the antiparallel α-actinin homodimer is shown in blue. The antiparallel monomer is shown in red. Sites formed by the R2 and R3 loops in each monomer lie in proximity with a two-fold symmetry on the same side of the α-actinin central rod.