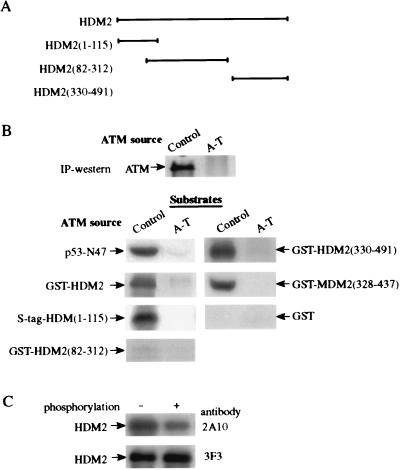
Figure 5.
In vitro phosphorylation of recombinant proteins by ATM. ATM was immunoprecipitated from control (L-40) and A-T (L-6) lymphoblastoid lines, and an immunoprecipitation-kinase reaction was performed for 15 min as described before (12). (A) Diagram showing various HDM2 fragments used as substrates (see Materials and Methods). The numbers in brackets denote the residues contained in each fragment. HDM2(1–115) was fused to an S tag, and all other fragments were produced as glutathione S-transferase (GST)-fusions. (B) In vitro phosphorylation of HDM2 and MDM2 fragments by ATM. Immunoprecipitated ATM was visualized by using immunoblotting. p53-N47, a polypeptide containing residues 1–47 of human p53 fused to the POU domain of the human transcription factor Oct-1 serving as a positive control substrate (12). Nonfused GST served as a negative control. (C) Reduction in immunoreactivity of recombinant HMD2 with the 2A10 mAb after in vitro phosphorylation by ATM. Full-length HDM2 was phosphorylated for 15 min by ATM and subsequently detected by immunoblotting with the antibodies 2A10 and 3F3.